Transradial artery access (TRA) has become a viable alternative to traditional transfemoral artery access (TFA) for many procedures performed by the interventionalist. Although this technique has been used for a number of years in the field of cardiology, the variety of intervention types and intervention locations were obstacles to the successful implementation of this technique within multiple fields of the vascular interventionalist, including vascular interventional radiology, interventional cardiology, vascular surgery and neurointervention. However, with the advent of a number of new devices specifically designed to optimise procedures from the radial approach, TRA for many common interventional procedures is now feasible and it is the preferred arterial access by patients compared to femoral.1–4 TRA techniques have specifically expanded into the areas of neurointervention and carotid and peripheral artery disease (PAD) interventions. At this time, all endovascular interventions (i.e. angioplasty, stenting, atherectomy and thrombectomy) are possible for viscera and coronary interventions and neurointervention. Most PAD interventions can be safely performed to the level of the superficial femoral artery (SFA) transradially; however, equipment size and length limit more peripheral work.1,5–8 Further research and device development supporting peripheral atherectomy and thrombectomy are underway in SFA interventions, given that TRA has been proven safe and feasible.9
Benefits of Transradial Artery Access
Before any new procedural technique can achieve mainstream implementation, there first needs to be proven benefit to the patient, the practitioner or the hospital system. As regards TRA, there is significant benefit in all three of these realms. Numerous percutaneous coronary intervention (PCI) studies demonstrated an improved safety profile of the TRA technique compared with TFA. The ACCESS, RIVAL, RIFLE-STEACS and MATRIX trials were randomised controlled studies comparing TRA with TFA for coronary intervention. These four trials involved more than 16,000 patients and demonstrated similar findings: that TRA leads to fewer bleeding-related complications, decreased all-cause mortality and fewer MIs when compared with TFA, without any difference in the incidence of stroke.8,10–13 In addition to decreased risk of major complications, there is a decreased risk of access-related complications when compared with femoral or brachial access.8 Access site complications are particularly relevant in the setting of anticoagulation and of thrombocytopenia. TRA has been shown to be a safe and feasible option with both thrombocytopenic and anticoagulated patients.13,14
Because of the obesity epidemic in the US, there is an increasing number of patients with large panniculus, which obstructs access for TFA. Patient obesity necessitates creative patient positioning to expose the femoral crease; requires the interventionalist to access atypically deep femoral arteries; and poses increased risk of post-procedure access site bleeding and infection. Radial artery access obviates many of these challenges in obese patients, given that the wrist has minimal fat compared with the femoral artery.
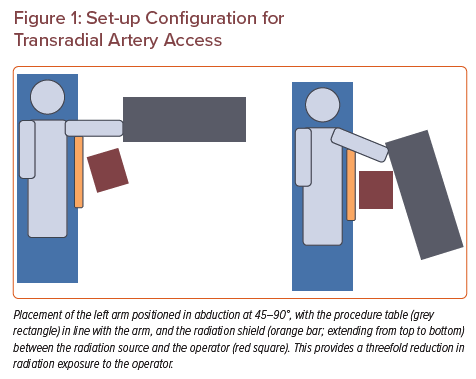
Additionally, TRA can be performed with the arm in abduction at 45–90° (Figure 1). In this configuration, the procedure table can be set up adjacent to the arm board as an extension of the patient table for convenient management of long devices. This positioning also increases the distance between the operator and the radiation source, with a threefold reduction in overall operator radiation exposure.3,15 If the arm is placed along the torso, TRA will provide no benefit from the radiation safety standpoint.16
TRA has been associated with improved patient and recovery staff satisfaction during post-procedure care. Multiple studies have demonstrated patient preference for TRA over TFA due to earlier ambulation, decreased post-procedure pain, decreased recovery time and less stringent recovery restrictions.1–3 In competitive medical markets, in which the healthcare industry moves toward patient consumerism, the patient’s experience is paramount. If patients have a more favourable recovery period, they are more likely to advocate for TRA and for minimally invasive vascular and interventional radiology (VIR) procedures.
The current economic pressure to reduce healthcare costs has elevated the importance of cost-effective treatments. Multiple publications have demonstrated conservative savings of US$250 per patient, which is in the realm of 10%. However, savings may be as high as US$1,621 per patient in cases of high predicted bleeding risk.17 In VIR, two retrospective studies evaluated cost savings of TRA compared with TFA; both found significantly decreased costs, one of them indicating a saving of US$100 per procedure.18,19 There is, perhaps, an even greater opportunity for cost savings in VIR, given that many VIR procedures can be performed on an outpatient basis. Because TRA patients do not require mandatory bed rest, a radial lounge recovery environment should be considered at high-volume centres. In this environment, TRA patients may be seated in recliners with ambulation privileges. This would decrease the amount of space required to care for each patient and decrease the demands on the nursing staff. The time required to monitor a TRA patient (1–2 hours) is also significantly shorter than for a TFA patient (2–6 hours). TRA offers the potential to optimise space and human resource utilisation.
Learning Curve
A study focused on the learning curve for interventionalists who are new to TRA demonstrated a threshold of only 20 cases to achieve equal procedure time, radiation dose and contrast use after implementation of TRA; and technical procedure success was equivalent for TRA and TFA regardless of TRA experience.20 Similar studies in PCI demonstrated a threshold for TRA experience of 30–50 cases; the difference is likely due to non-utilisation of ultrasound for access in the cardiology trials.21
Radial Access: Step by Step
Pre-procedure Work-up for TRA
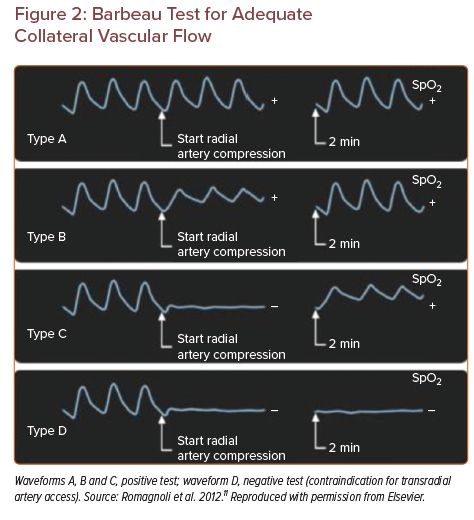
TRA work-up begins at the physical exam. A potentially limb-threatening complication of TRA is occlusion of the radial artery and ischaemia of the first and/or second fingers. However, in the appropriately triaged patient, digit ischaemia should never occur, even in the setting of radial artery occlusion (RAO), due to robust collateral circulation via a complete palmar arch vasculature. To assess for adequate collateral vascular flow, it is recommended to perform the Barbeau test. A pulse oximetry device, capable of displaying a plethysmographic venous waveform, is placed on the index or second finger. Initially the waveform should have normal amplitude. When manual compression is applied on the radial and ulnar arteries, the waveform should become flat. While compression is still applied to the radial artery, the pressure on the ulnar artery is released. The length of time to waveform return and the amplitude of the waveform determine the Barbeau waveform type: A, B or C (Figure 2). Barbeau type D refers to a waveform that does not return within a 2 minute window during compression of the radial artery.22 If the ulnar artery is the dominant feeding artery to the hand (larger diameter), then the Barbeau test should be performed in reverse on the ulnar artery using an equivalent technique. The Allen test is not recommended, due to the fact that the hand pigmentation in some patients might compromise visual evaluation of hand reperfusion.
In addition to the Barbeau test, it is recommended to use B-mode ultrasound to assess patency and to measure the anteroposterior (AP) diameter of the radial artery, inner to inner wall, in order to assure the compatibility of the radial artery diameter with the outer diameter of the necessary introducer sheath. Ideally this should be done in clinic or in the pre-procedure area to allow time for planning, room set-up and selection of adequate supplies. In emergency cases, these exams can be quickly performed when the patient is on the angiography table given that both do not take more than 1 minute in total. Colour Doppler can also be used if desired, but the probe should be angled to 30–60° for proper flow assessment.
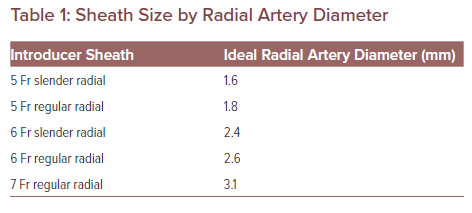
If the vessel measures >1.6 mm, a lower profile introducer sheath such as the 5 Fr Slender sheath (Terumo) may be introduced. A regular 5 Fr introducer sheath needs a radial artery AP diameter of at least 1.8 mm. If a 6 Fr Slender sheath, regular 6 Fr or a regular 7 Fr introducer sheath is used, they will require a 2.4 mm, 2.6 mm and 3.1 mm radial artery AP diameter, respectively (Table 1).
Caution should be used in patients with heavily calcified aortic arches, in those with a history of stroke and in the elderly. Theoretically, this population has increased stroke risk during TRA interventions. Meticulous technique is recommended to minimise neurological complications. Adequate TRA technique should include gentle catheter manoeuvres to minimise the risk of spasm and the use of fluoroscopy during the advancement of guidewire and catheter from the patient’s wrist until they reach the descending thoracic aorta. If the patient has an existing chest CT study, it should be reviewed prior to starting the procedure. Attention to these details will prevent inadvertent manoeuvres at the level of the aortic arch while obtaining access to the descending aorta.
TRA interventions are possible in patients with a tortuous aortic arch (especially in type 3), but slow catheter manipulation will likely be necessary during abdominal aorta catheterisations.
Basic Technique for Successful Outcomes
Correct TRA technique begins with proper room set-up and patient positioning. The patient should be positioned supine with arm abducted at 45–90° on an arm board. The pulse oximetry detector is placed at the tip of the first or second fingers, ipsilateral to the wrist used for TRA. After sterilising the arm, a towel is placed on the arm board and another one is rolled and placed underneath the wrist to hyperextend it. Tape is used across the palm and the distal arm board to prevent inadvertent movement of the arm during the procedure. At the beginning of the procedure, the fluoroscopy table must be broken so that the image detector is collimated over the patient’s forearm. The sterile procedure table should be set up in line with the patient’s extended arm to allow for catheters and wires to rest on the work surface (Figure 1).
Ideally, the radiologist should stand next to the patient’s thorax with both the ultrasound screen and fluoroscopy monitor near the patient’s head, across from the accessed arm. If this is not possible due to position of permanent room fixtures, the fluoroscopy or ultrasound screen can be placed on the opposite side of the patient’s torso. The radiation shield should be placed between the radiologist and the patient, just under the patient’s armpit. Decreased physician radiation exposure is a distinct advantage of the arm extended technique. The radiation protection tool that we typically use looks like a door on wheels. A good alternative is to position the ceiling-mounted shield above the angiography table skirt.
With all equipment in place, begin access by identifying the skin entry site for the needle over the radial artery approximately 1–2 cm proximal of the wrist crease. Under ultrasound guidance, infuse 0.5–1 ml lidocaine without epinephrine at this location. In the case of a very shallow radial artery (minimal amount of soft tissues between the skin and the anterior radial artery wall), an extra amount of lidocaine may be needed to create additional space to facilitate the arterial puncture . A short 21 G needle is held at 30–45° (shallower than femoral access) from the skin to access the radial artery under direct ultrasound guidance. When possible, single wall puncture is preferred, to decrease the incidence of posterior wall haematoma and vessel spasm.
Successful TRA will result in brisk backflow of blood from the needle hub and a 0.021 inch nitinol microwire is inserted (Seldinger technique). There should be minimal friction advancing the wire. Fluoroscopy should be used whenever there is resistance to advancement of the wire. In the case of difficulties advancing the wire, verify on ultrasound whether the needle tip terminates in the centre of the vessel, without double wall penetration or anterior wall tenting due to incomplete wall penetration. Once the wire is advanced towards the elbow, thread a hydrophilic radial sheath over the wire. Typically, no skin nick is needed due to the sharpness of the insert tip and smooth tapering tip.
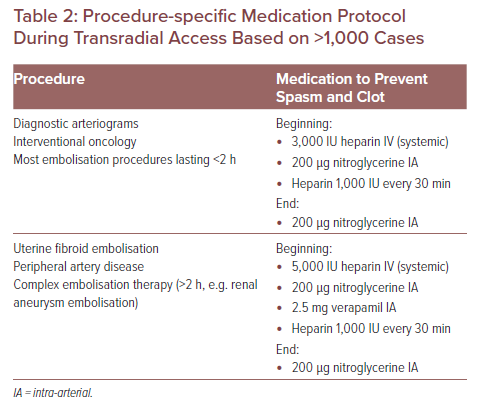
Attach a 10 ml saline syringe to the sheath side port and aspirate to confirm intravascular position with easy blood return. Subsequently, flush the sheath with the TRA cocktail (200 µg nitroglycerine, 2.5 mg verapamil and 3,000–5,000 IU heparin) diluted to a volume of 5 ml aspirated blood. Saline flush the sheath to deliver the full medication amount to the vessel. To prevent the hydrophilic introducer sheath from moving during the procedure, it is highly recommended to place a transparent dressing (Opsite, Smith+Nephew) on the top of it (covering the head of the side arm of the sheath). Intra-arterial heparin should be given immediately following successful arterial access at a dosage of 50 units/kg with 1,000 unit boluses every 30 minutes for procedures that last longer than 1 hour. Alternatively, heparin can be given IV by the procedure nurse with assessment for re-administration every 30 minutes (our preference). For procedures lasting longer than 2 hours, make dosage adjustments based on ACT level (2–2.5-fold higher than the baseline). A prospective randomised clinical trial demonstrated radial artery spasm at the end of the procedure (after guidewire and catheter removal).3 Therefore, our institutional intra-arterial medication regimen includes completion of anti-spasmodic treatment (Table 2).
Once TRA is successfully achieved, the radiologist can perform any of a large number of procedures from this location. Currently, TRA is primarily used for embolisation therapy, as well as in interventional oncology, visceral embolisation (splenic, renal, liver), gastrointestinal bleeding treatment, trauma, uterine fibroid embolisation and others.
The length of the upper extremity arteries introduces challenges of device selection when operating on patients taller than 1.88 m. A 110 cm diagnostic catheter will often be sufficient for thoracic cases and upper abdomen procedures, while 125–130 cm systems will be needed for angiography of the inferior mesenteric artery, pelvic embolisations and selective lower extremity run-off. For superselective catheterisations, a 2.0–2.8 Fr 150 cm long microcatheter will provide access to virtually every location in the body. For trauma of the lower extremities (especially distal thigh and calf), femoral access is probably the best choice due to catheter length.
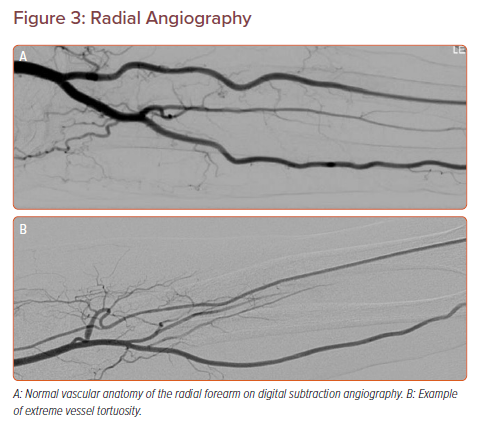
Anatomic variants of the upper extremities increase the difficulty level by introducing increased tortuosity between the radial artery and aorta. To avoid spasm and upper extremity vascular injury, it is recommended to use a J-tip and hydrophilic wire. Radial artery kinks or loops typically will be reduced and straighten out after a hydrophilic wire is advanced through (Figure 3). In order to understand the forearm arterial anatomy, we recommend performing forearm angiography in every patient (5–6 ml iodine contrast) to anticipate these challenges, soon after the sheath is introduced in TRA.
Navigating to the descending thoracic aorta can be challenging at times. Variations of aortic arch anatomy or aortic arch type 3 (elongated) might need special manoeuvres to overcome a difficult access. The simplest thing to do is to ask the patient to take a deep inspiration. Deep inspiration elongates the aorta and provides a better path for the guidewire. For more challenging anatomy, the whip manoeuvre may be used. The whip manoeuvre utilises a pigtail catheter positioned in the transverse arch with the open segment of the pigtail catheter facing the descending aorta. A 0.035 inch guidewire is advanced through the pigtail, which creates an exaggerated bend in the wire along the aortic arch and offers greater steerability to select the descending aorta. Alternatively, a microcatheter and microwire could be advanced coaxially through a 5 Fr diagnostic catheter to the descending aorta. Bending a long curve at the tip of the microcatheter may facilitate access to the descending aorta. The 5 Fr diagnostic catheter can then be advanced using the microcatheter and microwire as a guidewire. Increased resistance of the guidewire should be met with caution because this could indicate inadvertent catheterisation of the left internal mammary artery or that a right-sided aortic arch could be present with similar difficulty. When in doubt in any of these scenarios, perform contrast angiography to define the anatomy and rely on prior cross-sectional studies, which may be predictive of these challenges.
Access Closure
At completion of the procedure, remove the catheter over a wire to prevent vascular trauma to the aortic arch. Once the catheter is cleared from the introducer sheath, aspirate the sheath side port with a syringe to clear any blood clot that could have formed. Follow aspiration with a completion dose of intra-arterial anti-spasmodic cocktail of 200 µg nitroglycerine. Always make sure that the systolic pressure is above 100 mmHg. In the case of relative hypotension due to moderate sedation, consider the infusion of a bolus dose of saline to increase the blood pressure before the vasodilator is administered.
Patent Haemostasis
To prepare for sheath removal, place a 4 × 4 gauze under the sheath hub next to the skin entry site. Apply a radial artery compression device; the ideal device will allow adjustment to a pressure that achieves haemostasis at the arteriotomy without occluding the radial artery (patent haemostasis concept). The patency of the radial artery can be assessed manually by occluding the ulnar artery to perform a post-procedure reversed Barbeau test (occlude the ulnar artery to check if the radial artery is not excessively compressed by the balloon). Non-occlusive haemostasis decreases the incidence of RAO by 75% at 30 days.23 There should be a palpable pulse distal to the access site.
When the patient recovers in the post-procedure area, the patient should be monitored for bleeding at the access site. Monitor oxygen saturation of a digit distal to the access site, similarly to what was done in the procedure room. The pressure applied by the radial compression device can be systematically decreased per a standardised algorithm until the device can be removed. The patient should be monitored for an additional 30 minutes following removal of the device to ensure re-bleeding does not occur, at which point discharge is allowed. While in recovery, the patient will have less stringent positioning precautions compared with femoral access. The patient may be seated or semi-reclined with bathroom privileges. This small improvement in post-procedure care has a significant bearing on patient preference for radial access over femoral access.3
Radial Access Complications
The best way to minimise TRA complications is prevention. By implementing adequate pre-procedural patient selection and adhering to meticulous technique described above, many of these potential complications can be avoided. In the intra-procedure period, the two most common complications are radial artery spasm and RAO.
While radial artery spasm is temporary, it accounts for up to 38% of the technical procedure failures.24 As a result of spasm, the patient may communicate new cramping pain in the arm; the interventionist may sense increased resistance when manipulating the catheter. Multiple medications have been shown to relax arteries undergoing spasm, although there is no consensus opinion on which medication is most beneficial. Spasmolytic pharmacological options include nitroglycerine, verapamil or diltiazem. Our institution uses 200 µg nitroglycerine given at the onset of spasm, as well as prophylactically at the beginning and end of the procedure. Moderate sedation medications have also been shown to prevent and treat spasm, while also improving patient comfort.13 Alternatives to pharmacological intervention include application of warm compresses and inflation of a blood pressure cuff to 40 mmHg over the region of spasm followed by rapid deflation.
After the procedure, the most common complications are bruising and tenderness at the access site for 2–3 days.
Radial artery anatomic variants occur in approximately 13.8% of patients and are predictive of intra-procedure spasm.25 Radial forearm digital subtraction angiography should be performed at the initiation of therapy to identify challenging vascular anatomy early. The 360° radial loops and extreme radial artery tortuosity (Figure 3) were most predictive of procedure failure and occurred in 2.3% and 2% of patients, respectively.25 Meticulous catheter technique while navigating known areas of tortuosity will decrease incidence of spasm.
Post-procedure RAO is a potentially permanent complication. One meta-analysis suggests an incidence as high as 7.7% in the first 24 hours after the procedure.26 However, use of radial artery compression devices to provide non-occlusive haemostasis has decreased the incidence of RAO to less than 1%.27 Additionally, RAO is often clinically silent secondary to collateral flow. Early identification of RAO is an integral part of post-procedure care. A weak or absent pulse felt distal to the arteriotomy should initiate evaluation with Doppler ultrasound to confirm a diagnosis of RAO. Patients with confirmed RAO should receive systemic heparin.
Non-invasive treatment manoeuvres include 20 minutes of compression to the ipsilateral ulnar artery to help re-establish flow via the palmar arch.28 Local intra-arterial tissue plasminogen activator at the site of occlusion or balloon angioplasty may be given. Patients with RAO should be admitted overnight for observation and the arm should be wrapped to keep it warm and to promote hyperaemia. A total of 3–6 months of anticoagulation may be warranted and the patient should be scheduled for follow up in 30 days with Doppler ultrasound.
One aspect of TRA that increases the technical challenge, is the need to traverse the aortic arch for treatment. Many providers presume that manipulating a catheter in the aortic arch increases the risk of embolic or ischemic stroke. However, the incidence of neurologic complication following cardiac catheterisation remained unchanged at 0.2%, while the percentage of TRA procedures increased dramatically; this suggests that access site does not affect the incidence of stroke.29 Regardless, attention to prior cross-sectional imaging of the chest or neck is an important part of pre-procedure TRA work-up. Screening for challenging anatomy and areas of significant atherosclerotic disease, particularly in patients aged over 70 years or with a history of prior stroke, may prevent this complication. Imaging findings may alert the physician to special equipment needs or, in rare circumstances, indicate when the femoral approach is preferable.
Finally, there is a cluster of vascular complications that occurs in both TRA and TFA: post-procedural haematoma, access vessel rupture and access vessel pseudoaneurysm. Each of these complications occurs less frequently with TRA compared with TFA, and is more easily treated from TRA due to the easy exposure of the wrist.5 One exception is forearm compartment syndrome, which is encountered in only 0.4% of cases and is more likely to occur at the wrist than the groin in the setting of post-procedure haematoma.30
Staff Preparedness
An interventionalist is only as good as their surrounding staff. In implementing this new technique, consider including the nurses and radiological technologists in the education process. VIR staff are phenomenal resources for device selection and troubleshooting during challenging cases. Expert-led courses exist all around the world and the entire department could be included in this education from the beginning.
Conclusion
TRA is an emerging technique that can provide a safer and more convenient experience for the patient. Implementation of this new technique has unique advantages and pitfalls, but following best practice and using a meticulous technique can improve the quality of the service and increase patient satisfaction in endovascular interventions.