Transcatheter aortic valve implantation (TAVI) has emerged as the treatment of choice for patients with severe aortic stenosis (AS) deemed to be at high risk for surgical aortic valve replacement.1 Recent trials have suggested equipoise with regard to outcomes in patients with an intermediate risk, and clinical trials are underway in low-risk patients.2–8
The first TAVI procedure was performed by Alain Cribier in 2002 via an anterograde transeptal approach.9 Subsequently, the Placement of AoRTic TraNscathetER Valve (PARTNER) trial was published.10 This was the first randomised control trial of transfemoral TAVI, and compared it to the standard approach in patients with severe AS who were at high risk and inoperable. It demonstrated that TAVI reduced the risk of death (30.7% versus 49.7% at 1 year, p<0.001) and repeat hospitalisation (22.3% versus 44.1%, p<0.001) compared to standard therapy, despite a higher incidence of vascular complications (30.7% versus 5% at 30 days, p<0.001) and major stroke (5% versus 1.1% at 30 days, p=0.06).
Based on the results of PARTNER 1, the transfemoral route became the default access site for TAVI. Initially, this was achieved via surgical cutdown, which led to a correspondingly higher rate of vascular complications. The PARTNER 2 trial subsequently confirmed the utility of TAVI in intermediate-risk patients with AS, randomised to either TAVI or surgery.11 Of the 1,011 patients randomised to TAVI in this trial, the majority (76.6%) underwent transfemoral TAVI, with the remainder undergoing a transapical (17.2%) or transaortic approach (6.2%). In this trial, the transthoracic approach was not found to be superior to surgical aortic valve replacement. In the transthoracic cohort, TAVI did not result in a lower rate of death from any cause or disabling stroke than surgery. This confirmed the primacy of the transfemoral approach for TAVI where it is possible in these patients.
This article provides a comprehensive overview of the current options for alternative vascular access in TAVI. We will also touch on the clinical rationale for using the various access routes and, where possible, discuss the best available current evidence and suggest areas in need of further clinical investigation.
Current Practice in Transcatheter Aortic Valve Implantation
The transfemoral route (TF TAVI) is the most commonly used access type for TAVI.12–14 The first-generation devices in 2005 required a femoral cutdown and the use of up to 24 Fr sheaths. Sheath sizes have decreased considerably since and current devices use 14–16 Fr sheaths. This has negated the need for surgical cutdown in the majority of cases, reduced vascular complications and increased the proportion of patients eligible for TF TAVI.
There has been much progress with regard to simplification of the TF TAVI in recent years. This includes a move toward conscious sedation rather than general anaesthesia, percutaneous femoral access rather than surgical cut down, temporary pacing through the left ventricular guidewire instead of inserting a right ventricular temporary pacing wire through a femoral vein sheath, dedicated vascular closure devices, operating without transoesophageal echo guidance and post-procedure care plans designed to promote early ambulation and discharge.15–20 This modern, ‘minimalist’ TAVI approach is fast becoming the standard of care for straightforward cases.
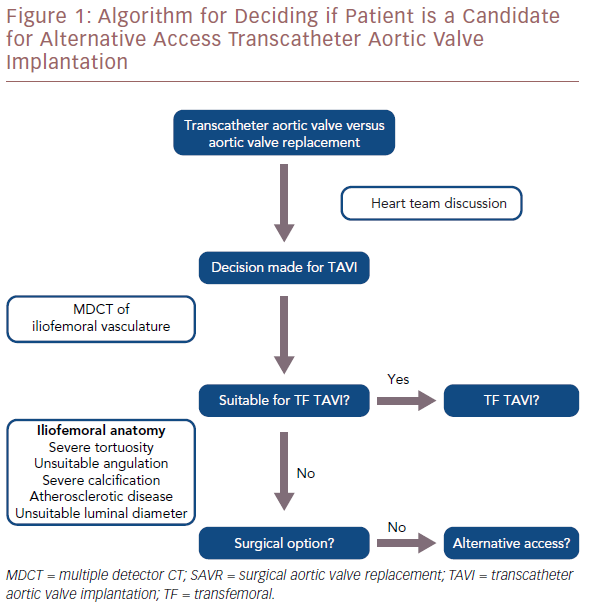
However, because of severe atherosclerotic disease, tortuosity, calcification or angulation, transfemoral access may not be feasible in around 10–15% of cases.14 A luminal diameter >6 mm is usually required. Severe calcification of the peripheral vasculature and coarctation of the aorta are also considered contraindications to the transfemoral route. Similarly, previous surgery or stenting in the aorta, iliac or femoral arteries may represent a relative contraindication. In such cases, several alternative access options are now available to the operator (Figure 1).
Alternative Access Options
Rationale
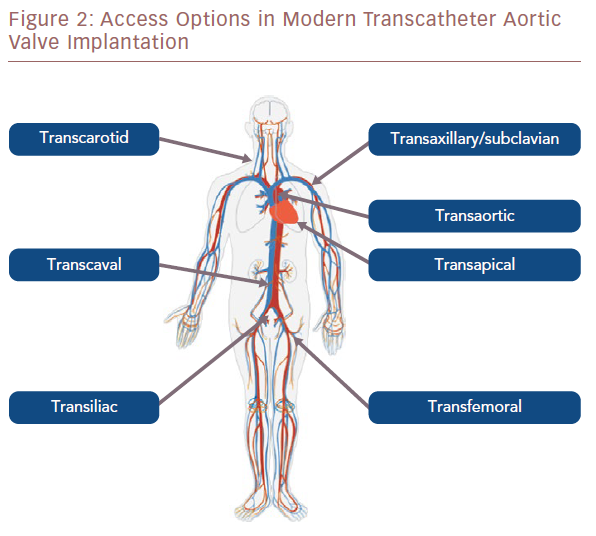
Percutaneous transfemoral access is considered the safest approach for TAVI and has become standard care for the majority of cases, as discussed above. Other vascular access sites (direct aortic, axillary, carotid and subclavian) also use the retrograde approach, but most require a surgical cutdown. Some of these approaches can now be performed percutaneously, but this method is still in its relative infancy in the majority of centres (Figure 2).
Recently, transcaval TAVI has been described, using femoral vein access. A novel suprasternal approach has also been proposed although data on this is currently limited.21 Each access route is discussed below in more detail.
Transapical and Transaortic Access
Initially, transapical (TA) TAVI was the preferred alternative access route for TAVI in patients with unsuitable femoral access. It is the only anterograde approach for TAVI. It requires general anaesthesia, a mini-thoracotomy and direct puncture of the left ventricular apex. This allows for easy valve crossing and excellent control of the valve position during implantation. The direct puncture of the apex may result in reduced left ventricular function, myocardial necrosis or myocardial stunning.
In the transthoracic cohort of the PARTNER 2 trial, the majority of whom had TA TAVI, outcomes were not superior to surgical aortic valve replacement.11 However, the trial was not powered for this subgroup analysis. The majority of experience in TA TAVI is in balloon expandable valves.
The use of TA TAVI has reduced over time. This is mainly for two reasons. First, smaller sheath sizes for TF access and greater operator experience have increased the proportion of patients suitable for TF TAVI. Second, the development of multiple other, less invasive access routes has expanded the modern operators’ armamentarium and concern over the higher complication rate associated with TA TAVI has led operators to prefer alternative access routes.22
The transaortic (TAo) approach was also used in the PARTNER 2 trial, although in a smaller number of patients (n=65, 6.4% of total).11 Access is gained via a mini-thoracotomy or partial sternotomy. The procedure is performed under general anaesthesia. Cardiopulmonary bypass is generally not required. Once aortic access is gained, the valve can be deployed via a retrograde approach.
Bapat et al. described their experience of TAo TAVI using a partial upper sternotomy in 17 patients using the balloon-expandable SAPIEN (Edwards Lifesciences) valve in 2012. They reported 30-day mortality of 4.3% versus 7.7% in the corresponding TA cohort and no significant differences in procedural complications.23
TAo TAVI is contraindicated in patients with a heavily calcified or atheromatous ascending aorta, vein grafts with high origin, or anatomical variations that might prevent good coaxial prosthesis deployment (i.e. pectus excavatum). It is preferred to TA TAVI in patients with severe pulmonary disease compromising the pleural space, severe systolic dysfunction, a small left ventricular cavity and a thin left ventricular wall.
Amrane et al. performed a meta-analysis on TAo TAVI. They included 16 studies, all of which were observational and single arm. They reported a major vascular complication rate of 31%, pacemaker implantation rate of 11.7%, a 9.9% 30-day mortality rate and a 3.7% stroke rate.24
Trans-subclavian/Transaxillary
The subclavian approach was initially performed after a surgical cutdown with isolation and preparation of the artery by a vascular or cardiothoracic surgeon. More recently, a true percutaneous subclavian approach has been discussed. Petronio et al. described their initial multicentre experience of 54 cases, all of which used the self-expanding CoreValve (Medtronic) prosthesis.25
Pre-procedural CT scanning and angiography were performed to assess the diameters, tortuosity and calcification of the left axillary and subclavian arteries. The presence of a left internal mammary artery graft was not considered a contraindication as long as the subclavian artery was larger than 7 mm and free from atherosclerotic disease. Patients were considered ineligible for the procedure if the vessel diameter was <6 mm, if the vessel was heavily calcified or tortuous, or if there was tight subclavian stenosis that was not amenable to balloon angioplasty.
After surgical isolation of the artery, arterial access was obtained using the Seldinger technique. Initially a 6 Fr sheath and subsequently an 18 Fr sheath were inserted into the artery, then TAVI was performed using the standard technique. Procedural success was achieved in 100% of subclavian cases, compared with 98.4% of transfemoral TAVI cases (p=0.62). Procedural duration was longer in the surgical subclavian access group (120 minutes versus 70 minutes; p<0.0001).
Intra-procedural mortality was 0% in the subclavian group and no specific complications were reported related to subclavian access. General anaesthesia was used for the majority of cases but, in centres with more experience of the procedure, local anaesthesia was also used. The left subclavian artery was used preferably as it allows for a more favourable orientation of the CoreValve delivery system through the aortic annulus. Right subclavian access requires that the 18 Fr sheath remains distal to the origin of the right internal carotid artery to avoid hypoperfusion of the brain. Valve implantation can also be more difficult due to the wider angle between the delivery catheter and the axis of the ascending aorta. Petronio et al. reported that subclavian access is feasible in patients with a pacemaker in situ as the surgical cutdown does not interfere with the pacemaker system.25
The US Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) Registry reported on 627 patients undergoing trans-subclavian and transaxillary TAVI using the SAPIEN 3 (Edwards Lifesciences) prosthesis.26 Procedural success was achieved in 97.9% of cases with a 30-day all-cause mortality of 4.4% and a major vascular complication rate of 3.1%. The stroke rate was 5.4%. The STS/ACC TVT registry did not differentiate between the trans-subclavian (supraclavicular) and transaxillary (infraclavicular) approaches. A fully percutaneous technique was used in 95 cases (15.2%). In the Nordic Aortic Valve Intervention (NOTION) trial, trans-subclavian access was used in only 3.5% of cases (n=5).2
Transaxillary TAVI was also initially performed via surgical cutdown. However, as with trans-subclavian access, fully percutaneous transaxillary TAVI can also be performed. One study of 100 consecutive patients in two centres demonstrated a device success rate of 95% with 0% major and 11% minor access site complications.27 Mortality rates were 6% at 30 days and 14.8% at 1 year. Smaller published series have demonstrated similar results.28,29
Transcarotid
Mylotte et al. described a large series of transcarotid TAVI, with 96 patients undergoing the procedure in three high-volume TAVI centres in France over 4 years (2009–2013).30 The patients included in this registry had small-calibre, heavily calcified, tortuous or stenotic iliofemoral anatomy or significant descending aortic pathology. Exclusion criteria in this study, which indicated patient was not suitable for a transcarotid approach, included significant (50%) common or internal carotid stenosis and congenital variants of the aortic arch.
This series primarily used the CoreValve (92.7%); the Sapien valve was used in the remaining 7.3% of cases. Cardiac tamponade occurred in 4% of cases and there were no conversions to surgical aortic valve replacement. No major bleeding or vascular complications related to the access site occurred. Mortality at 30 days was 6.3% (n=6) with half of these being procedural deaths (n=3). There were no in-hospital strokes but there were three transient ischaemic attacks (3.1%).
One of the most noticeable advantages of the transcarotid approach is that it does not require a thoracotomy or mini-sternotomy. As such, it may be preferable in patients with significant respiratory comorbidities or those who have had a prior sternotomy. In this cohort, the carotid artery was exposed via a small supraclavicular incision. The artery was dissected and clamped proximally and distally. The artery was then accessed percutaneously, allowing the transcatheter heart valve to be implanted. The arterial access site was repaired surgically after the delivery sheath had been removed.
In this study, multislice CT was used to determine the dimensions of the carotid, subclavian and vertebral arteries with a minimal luminal diameter of 7 mm and above considered appropriate for trans-carotid access. Performing magnetic resonance angiography of the circle of Willis before the procedure is essential to determine if adequate collateral cerebral blood flow is present for potential transcarotid access. This was carried out in all cases in this series. The left common carotid artery is usually preferred as it provides superior coaxial alignment and was used in 88.5% of patients in this series.
TAVI via the carotid route is certainly a viable option. However, recent meta-analyses and systematic reviews have suggested that the available data limit formal meta-analysis and, as such, they draw no firm conclusions regarding the safety and efficacy of this method.31,32
Transcaval
The transcaval approach is the most novel alternative access route for TAVI. It involves femoral vein access with crossover to the abdominal aorta from the inferior vena cava. This is achieved by electrifying a caval guidewire and advancing it into an aortic snare.
Importantly, like transcarotid TAVI, it avoids the morbidity of transthoracic approaches. Periprocedural CT scanning is paramount to ensure there is a suitable non-calcified area of abdominal aorta to allow wire crossover from the inferior vena cava. This area of aorta should also be free of any important arterial branches, such as the renal artery, renal vein and aorto-iliac bifurcation, as a covered stent may be required as a bailout strategy if there are bleeding complications during the procedure.
A microcatheter is delivered into the descending aorta and a stiff guidewire is introduced. Once crossover is achieved, the TAVI introducer sheath can be introduced in the usual manner and the valve implanted using a retrograde approach. A nitinol cardiac occluder device is used to close the iatrogenic aortocaval fistula after valve implantation.
The largest cohort, described in the literature by Greenbaum et al., consists of 100 patients who were ineligible for femoral artery access and had a high or prohibitive risk from transthoracic access.33 Device implantation was successful in 99 patients. They reported a 30-day survival of 92%, a life-threatening bleeding rate of 7% and a major vascular complication rate of 13%.
While this technique has certainly been demonstrated to be feasible, it is probably best reserved for use in experienced, high-volume specialist centres at present, but may become more commonplace in future. A recent meta-analysis concluded that the initial evidence is encouraging but further prospective studies are probably required before any firm conclusions can be made.34
Deciding on an Alternative Access Route
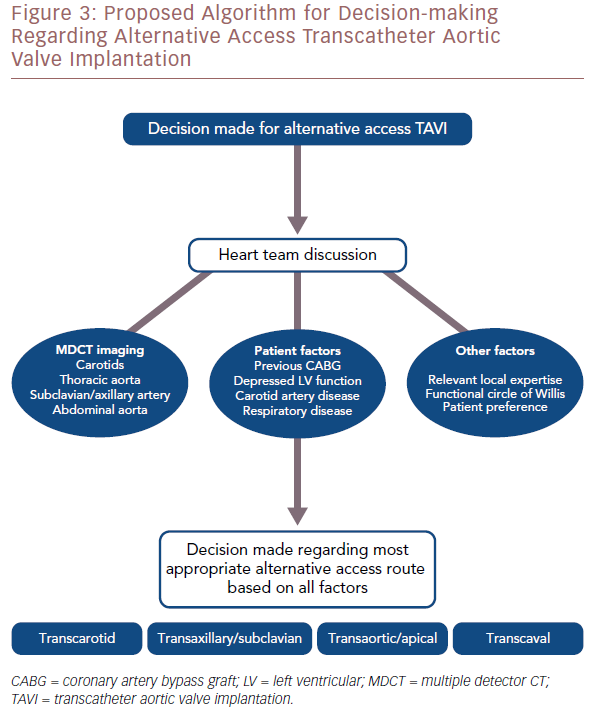
Multi-detector CT scanning plays a vital role in determining the most appropriate access route. It can give information on luminal diameter, calcific vessel load and tortuosity of the relevant vasculature. For TF TAVI, it allows practitioners to assess the iliofemoral vessels bilaterally to determine if a patient is suitable. If transcaval access is being considered, CT scanning can identify a suitable area on the right aortic wall for passage of the transcatheter aortic valve replacement sheath from the inferior vena cava to the abdominal aorta. For transcarotid TAVI, it provides valuable information on the subclavian, carotid and vertebral arteries. Imaging experts play an important role in the heart team discussion and can help guide operators toward the most appropriate access route.
In general, vascular access site complications are a common cause of significant morbidity and mortality after a TAVI procedure. A key role of the heart team should be to identify the access route that will minimise this risk. If alternative access is considered, a vascular surgeon should be part of the heart team to identify potential risks associated with the access routes and assist in the decision-making (Figure 3).
Conclusion
Since its inception, TAVI has undoubtedly revolutionised the treatment of aortic stenosis. Enormous strides have been made with regard to patient and device selection, pre-procedural planning and procedural simplification. This has resulted in improvements in patient outcomes and increased operator confidence with regards to taking on more complex cases. Unsuitable iliofemoral anatomy no longer precludes patients from undergoing TAVI and physicians have become more comfortable with alternative access routes. Without intervention, these patients have a poor prognosis with a mortality rate of around 50% at 2 years.35
While much of the published data on alternative access TAVI shows promising results, the majority of this is registry data rather than randomised controlled trials. TF TAVI remains the safest access route and should be considered in the majority of cases.
However, in unsuitable patients, different access routes have been shown to be safe and feasible. The challenge is to choose the best alternative access route for the individual patient based on their vascular anatomy and comorbidities, choice of valve and the local institutional skill set.