The development of atherosclerotic disease is an evolving process that may present as chronic to subacute or acute.1 Arterial thrombosis – the formation of obstructive thrombus – may be part of the atherosclerotic process and may occur spontaneously due to abnormal balance of haemostasis/thrombosis and the fibrinolytic system or in response to vessel injury, for example during balloon angioplasty.1,2
Balance is maintained by the complex interactions among the coagulation cascade, platelets and the fibrinolytic system. Anticoagulants are effective in inhibiting the activity or synthesis of coagulation factors, hence preventing the formation of a fibrin clot. Anticoagulants have been approved for prevention and treatment of deep venous thrombosis/pulmonary embolism (DVT/PE) or AF since the 1940s and their use for people with peripheral artery disease (PAD) has been tested over the past decade.
This review analyses the use of anticoagulants in patients with lower limb artery disease with claudication or critical limb ischaemia. Papers included in the literature search were selected using the keywords anticoagulant/anticoagulation, direct anticoagulant, thrombosis and peripheral artery disease, published from 2000 onwards. Studies investigating anticoagulants versus antiplatelets were included, otherwise studies focusing only on antiplatelets in PAD or anticoagulation which did not report PAD outcomes were excluded from this review.
Old Anticoagulants Used for Peripheral Arterial Disease
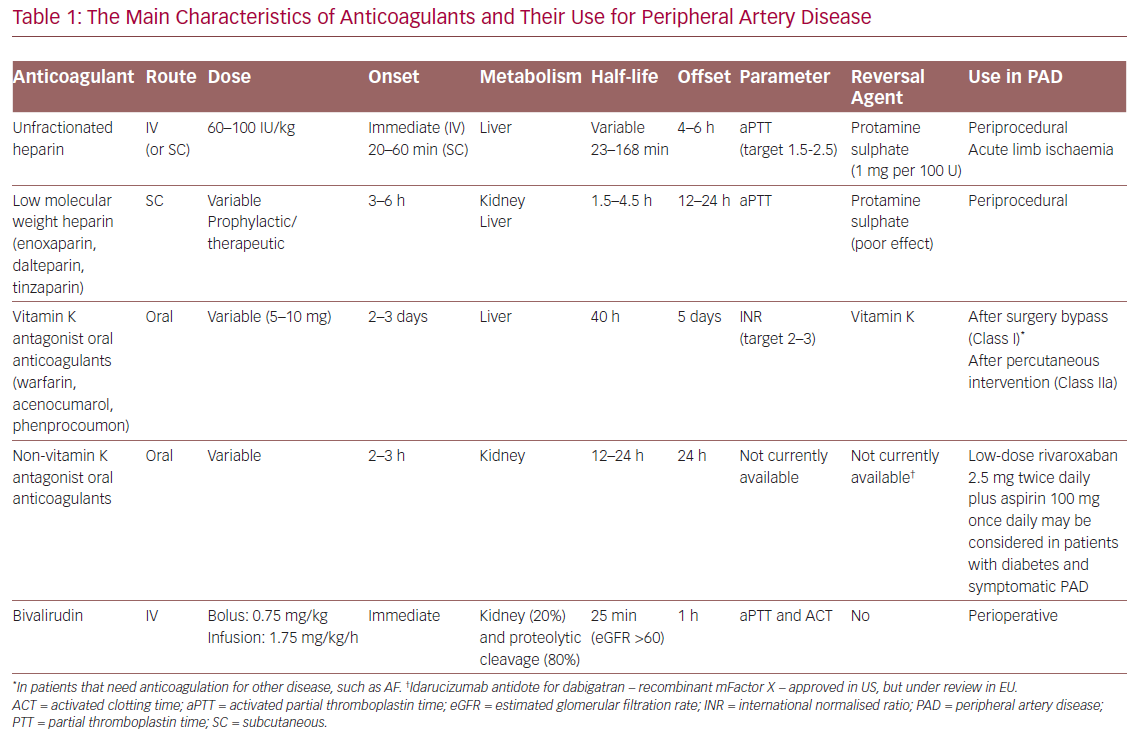
Up until the 1990s, two types of anticoagulants – heparins and vitamin K antagonists (VKAs) – were considered standard for antithrombotic therapy. Their limitations, including major bleeding, unpredictable pharmacodynamic responses and immunogenicity, sparked the development of low molecular weight heparin (LMWH) and heparin oligosaccharides, such as fondaparinux. Heparins and the common VKA warfarin both have multiple targets in the coagulation system.3
Heparin takes effect rapidly via IV infusion, while warfarin acts slowly via oral administration. Both drugs have a narrow therapeutic window and large variation in responses among patients. In rare cases they may cause serious complications, such as heparin-induced thrombocytopenia and coumarin-induced necrosis.3 Thus, clinical monitoring and dose adjustment are required during treatment.
Heparins
The heparins used for the clinical management of PAD are unfractionated heparin (UFH) and LMWH and they are mainly used in a perioperative context. UFH is associated with immediate initial bioavailability, rapid clearance and is usually administered by continuous IV infusion. The platelet count should be monitored after the prolonged administration (>4 days) of heparin due to the risk of heparin-induced thrombocytopenia.4
For decades, UFH has been the anticoagulant of choice for longer-term treatments. Due to its pharmacodynamic drawbacks, it has been replaced mainly by LMWH and also by fondaparinux. UFH is currently used with an IV bolus for periprocedural anticoagulation in endovascular and surgical procedures or in cases of acute limb ischaemia at higher doses in continuous infusion. In the latter situation, full anticoagulation should be initiated as soon as possible and continued until thrombolysis is started, as it has been shown that the risk of amputation is related to time to therapy.5
A commonly used intraoperative IV dose is 100–150 units/kg; intra-arterial use, which although commonly used in practice, remains off-label.5 Thompson et al. reported intraoperative UFH can protect against perioperative MI in abdominal aortic aneurysm surgery.6 Otherwise low LMWHs are not widely used intraoperatively because of their long duration of action that cannot be completely reversed with protamine.
LMWHs, such as enoxaparin, dalteparin and tinzaparin, may be used either for short- and long-term therapy in patients with PAD undergoing endovascular or surgical interventions and is mainly administered subcutaneously. Therapeutic dosing (often dosed by weight) is maintained at 12-hour intervals, whereas prophylactic dosing is kept at 24-hour intervals. LMWH has a much higher bioavailability than UFH (>90% of the administered dose) and a more predictable dose response. However, the use of LMWH in patients with renal failure or morbid obesity is complicated by less predictable clearance kinetics.3,5
A study investigating the use of LMWH in patients undergoing endovascular interventions found that perioperative enoxaparin administered in low-risk and high-risk patients for reocclusion, is a safe and effective treatment in regard the risk of bleeding and short-term target lesion reocclusion with no reocclusion in low-risk patients (n=44) versus 3.6% in the high-risk group (n=140) at 180 days.7
A randomised controlled trial (RCT) of patients undergoing endovascular interventions for PAD (Fontaine stage IIb–IV) found that the use of enoxaparin as a single weight-adapted bolus of 0.5 mg per kg body weight is superior to UFH with regard to safety and efficacy. The primary composite endpoint of bleeding, occlusion or reintervention occurred in 10.5% of cases for UFH versus 2.5% for enoxaparin (p<0.05).8
In patients taking aspirin, the risk of bleeding was higher with UFH compared with enoxaparin. Otherwise in patients with uncomplicated claudication, a Cochrane review found that there is no definite role of heparin (UH and LMWH) in preventing atherothrombotic events.9
Warfarin and Other Coumarins
Worldwide, warfarin is the most used drug for non-PAD antithrombotic therapy, but in Europe, acenocumarol and phenprocoumon are also prescribed.3 These have a shorter half-life than warfarin, which could be an advantage in cases of bleeding. Warfarin is currently approved for use in the treatment of AF and thrombosis prevention for people with coagulation disorders. A number of studies have also investigated the use of these oral anticoagulants in patients with PAD.
A Cochrane systematic review found that oral anticoagulants may have a potential role in the treatment of patients with intermittent claudication (IC).7 Their antithrombotic action might influence the progression of disease and the acute complications of thrombosis superimposed on chronic atherosclerotic lesions. However, it concludes that a clear benefit of anticoagulants for IC cannot be established due to the low methodological quality of the available studies. Among trials and studies that tested oral anticoagulants in PAD, the Warfarin and Antiplatelet Vascular Evaluation (WAVE) and the Dutch By-pass Oral anticoagulants or Aspirin study (Dutch BOA) are the landmark trials, as they are the largest and best designed studies.10–12
The WAVE trial enrolled both claudicant and critically ischaemic patients, randomisation was warfarin or acenocoumarol plus antiplatelet therapy versus antiplatelet alone. In a cohort of 2,161 patients, anticoagulation or antiplatelet therapy compared with antiplatelet therapy alone did not reduce major adverse cardiovascular or cerebrovascular events (MACCE; 12.2% versus 13.3%, respectively; RR 0.92; 95% CI [0.73–1.16]) or a composite endpoint of MACCE and severe ischaemia of the peripheral or coronary arteries (15.9% versus 17.4%, respectively; RR 0.91; 95% CI [0.74–1.12]). Life-threatening bleeding was increased with anticoagulation (4.0% versus 1.2%; RR 3.41; 95% CI [1.84–6.35]).
The Dutch BOA trial studied the benefit of phenprocoumon or acenocoumarol versus daily 80 mg aspirin in 1,546 patients with an infrainguinal venous bypass as a prespecified subgroup analysis with a mean follow-up of 21 months. The target international normalised ratio range was kept at 3.0–4.5 in patients on VKA therapy. Results showed not only that VKAs were superior to aspirin in the prevention of graft occlusion (HR 0.69; 95% CI [0.54–0.88]), but also increased the risk of major bleeding.
Although it did not reach statistical significance, the rate of amputations appeared to be lower in patients receiving VKAs after a venous bypass (6.6% versus 9.3%; HR 0.72; 95% CI [0.5–1.01]), and the composite outcome of vascular death, MI, stroke or amputation (HR 0.89; 95% CI [0.75–1.06]) was also reduced.
A re-analysis of the Dutch BOA trial found that aspirin alone instead of VKAs in patients with a high risk of bleeding would result in fewer non-fatal haemorrhages, but would increase ischaemic events and graft occlusions.13
A meta-analysis including eight trials comparing antiplatelet versus anticoagulation, confirmed that the addition of an anticoagulant to an antiplatelet drug leads to an increased patency rate of vein bypass at the cost of a higher incidence of bleeding complications.10 Contrary patients undergoing prosthetic bypass grafts benefitted more from antiplatelet monotherapy than anticoagulation.
In the WAVE trial, patients with known risk factors, such as long-term use of non-steroidal anti-inflammatory drugs, previous gastrointestinal bleeding or recent stroke were excluded from participation to minimise the risk of bleeding.11 Despite this, nearly 30% of patients discontinued oral anticoagulation therapy during follow-up, many (126 of 319) because of bleeding episodes.
On the basis of those results, international guidelines recommend the use of oral anticoagulants for PAD only if there is a concomitant condition, such as AF or mechanical aortic valve, that requires anticoagulation.14 This is because the evidence of the benefit of oral anticoagulants in reducing major adverse limb events or revascularisation in PAD is weak and a higher incidence of bleeding was found when compared with antiplatelet therapy.
Direct Oral Anticoagulants
Direct oral anticoagulants (DOACs) were introduced in 2008 and are used for multiple thromboembolic disorders as they have advantages over existing agents. They are also known as non-VKA OACs or novel oral anticoagulants and offer reliable levels of anticoagulation and lower rates of intracranial haemorrhage and life-threatening or fatal bleeding compared with VKAs. They are alternatives to LMWH in a perioperative setting for venous thromboembolism (VTE) prophylaxis and therapy, and to VKAs for longer-term therapy.3,10 DOACs have predictable pharmacokinetic/pharmacodynamic effects, which means that routine coagulation monitoring for titration and maintenance is not required.
Most DOACs are direct inhibitors of Factor Xa or thrombi which are responsible for the coagulation cascade. The new antiplatelet agents ticagrelor and vorapaxar act directly on specific platelet receptors, such as P2Y12, inhibiting the platelets’ aggregation.15 Essentially acting on different pathways, all those agents reduce the risk of thrombus formation. Several studies and trials are investigating the effectiveness of these new anticoagulants in improving long-term outcomes in patients with PAD and reducing major adverse limb events.15
One of the earlier trials was the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial which assessed the efficacy of rivaroxaban ± aspirin versus aspirin alone.16 The trial enrolled 27,395 patients at 602 centres worldwide with stable atherosclerotic vascular disease (CAD, PAD or both), who were randomised to three arms (aspirin 100 mg daily versus rivaroxaban 5 mg twice daily versus aspirin 100 mg daily plus rivaroxaban 2.5 mg twice daily. In a sub-cohort of 7,470 patients affected by PAD (55.2% symptomatic limbs, 19.1% with low ankle-brachial index and the rest with carotid disease), rivaroxaban 2.5 mg twice daily plus aspirin compared with aspirin alone reduced MACCE (5.1% versus 6.9%; p=0.005), major adverse limb events (MALE; 0.9% versus 2.4%; p=0.004), MALE components of acute limb ischaemia (0.8% versus 1.4%; p=0.04), and major amputation (0.2% versus 0.7%; p=0.01). The combination of rivaroxaban plus aspirin increased bleeding compared with aspirin alone. Bleeding was mainly gastrointestinal (1.6% versus 0.7%; p=0.03) with few intracranial (0.2% versus 0.4%) or fatal haemorrhages (0.2% versus 0.1%). The authors concluded that rivaroxaban 2.5 mg twice daily administered with aspirin should be considered for the prevention of atherothrombotic events only in adult patients with CAD or symptomatic PAD at high risk of ischaemic events.
Considering this study, the European Society of Cardiology (ESC) guidelines on diabetes and the European Society for Vascular Medicine (ESVM) guidelines recommend that low-dose rivaroxaban 2.5 mg twice daily plus aspirin 100 mg once daily may be considered in patients with symptomatic lower extremity PAD but without a high risk of bleeding (recommendation Class IIb for ESC guidelines and Class IIa for ESVM guidelines, level of evidence B).17,18 The National Institute for Health and Care Excellence also released a similar recommendation for low-dose rivaroxaban twice daily in patients with CAD or PAD at high risk of ischaemic events, including acute limb ischaemia and amputation.19
The latest trial, Efficacy and Safety of Rivaroxaban in Reducing the Risk of Major Thrombotic Vascular Events in Subjects With Symptomatic Peripheral Artery Disease Undergoing Peripheral Revascularization Procedures of the Lower Extremities (VOYAGER PAD), confirmed the positive results of rivaroxaban plus aspirin versus placebo plus aspirin in patients who have undergone revascularisation.20 In this trial, 6,564 patients were recruited and blindly randomised with 3,286 assigned to the rivaroxaban group and 3,278 assigned to the placebo group. Results showed acute limb ischaemia and limb loss for vascular causes were significantly lower in the rivaroxaban group at 28 months (acute limb ischaemia: 4.7% versus 6.9%; major amputation: 3.1% versus 3.5% for rivaroxaban versus placebo group, respectively; p<0.001). The unplanned index-limb revascularisation for recurrent limb ischaemia rate was also lower in the rivaroxaban group (17.8% versus 20%; p=0.03).
Primary safety outcome thrombolysis in MI (TIMI), major bleeding incidence (fatal bleeding, intracranial haemorrhage, a decrease in haemoglobin level of ≥5 g/dl, or a decrease in haematocrit of ≥15%) was not different between the two groups. The secondary safety outcome of major bleeding, defined by International Society on Thrombosis and Haemostasis (ISTH), was significantly more frequent in the rivaroxaban group versus placebo (5.94% versus 4.06%; p=0.007).
Another RCT – Edoxaban plus aspirin versus dual antiplatelet therapy in endovascular treatment of patients with peripheral artery disease (ePAD) – investigated the safety and efficacy of edoxaban to prevent loss of patency following endovascular treatment.21 This trial compared the use of edoxaban plus aspirin versus conventional treatment with dual antiplatelet therapy (clopidogrel and aspirin) in 275 symptomatic patients (29% who were claudicant). Lesions were mainly located in the superficial femoral artery and stents were used in about 54% of patients in each group.
There were no major or life-threatening bleeding events in the edoxaban group while there were two major and two life-threatening bleeding events in the clopidogrel group. After 6 months of observation, there was a lower incidence of restenosis/reocclusion with edoxaban compared with clopidogrel, although this was not statistically significant (30.9% versus 34.7%; RR 0.89; 95% CI [0.59–1.34]; p=0.643). Although there is evidence of a real advantage of using edoxaban over clopidogrel, the authors concluded that larger and longer-term trials should confirm those findings.
Other Direct Anticoagulants
Other direct anticoagulants can inhibit the thrombin directly rather than acting on Factor Xa and they are mainly dabigatran (univalent) and bivalirudin (BIV) which is bivalent and IV administration only. Dabigatran is a DOAC that is mainly used in the prevention of stroke/embolism in patients with non-valvular AF, or VTE prevention in patients undergoing hip surgery.3 There is a paucity of evidence about the use of this DOAC for PAD with only an observational analysis by Lopes et al. finding dabigatran comparable to the other DOACs in terms of stroke/MI/all-cause mortality rates, being lower than warfarin.22 However, no specific results were focused on the long-term effect on peripheral artery atherosclerosis.
In regard to BIV, RCTs for percutaneous coronary interventions have shown that BIV had a significant advantage of decreasing procedural bleeding over UFH due to its very short half-life.23 A contemporary systematic review has analysed the efficacy of BIV versus UFH in peripheral endovascular interventions excluding intracardiac procedures.24 Generally, patients who received BIV had significantly reduced risks of MACCE, net adverse clinical events, major and minor vascular complications, compared with the unfractionated UFH group. Patients who received BIV had a lower but non-significant odds of major bleeding (OR 0.72; 95% CI [0.47–1.11], minor bleeding (OR 0.74; 95% CI [0.55–1.00]) and transfusion.
From the enrolled studies that reported postprocedural limb amputation, the difference between BIV and UFH was not significant, suggesting that the incidence of stent thrombosis may be similar between BIV and UFH. Given the equivocal results, the authors conclude that BIV may be chosen solely as an alternative procedural anticoagulant to UFH.
What’s Next?
Large clinical studies are ongoing in the US and should provide meaningful information on treatments for people with PAD. The Best Endovascular Versus Best Surgical Therapy in Patients With Critical Limb Ischemia (BEST-CLI) trial, is an open-label RCT plans to enrol 2,100 patients at 120 centres in the US and Canada.25 A concurrent registry is planned and will capture real-world antithrombotic therapy in patients with critical limb ischaemia.
Another study named DUAL Pathway Inhibition to Improve Endothelial Function in Peripheral Artery Disease (DUAL-PAD; NCT04218656), is still recruiting and will provide information about endothelial function in patients on low-dose rivaroxaban plus aspirin (100 mg) versus aspirin (100 mg) alone.
Conclusion
LMWH represents the leading drug for perioperative management of patients with PAD. Otherwise oral anticoagulation with warfarin/cumarols is mostly used in patients who need this for AF or DVT and have symptomatic PAD. A certain benefit may be to maintain patency of lower-limb bypass grafts, considered at risk.
In regard to DOACs, there is now level 1b evidence regarding the long-term benefit of low-dose rivaroxaban plus aspirin that is recommended by the most recent ESC guidelines for people with diabetes.
Finally, the additional risk of bleeding over aspirin alone still remains. The appropriate anticoagulation regimen for patients with PAD should be decided by balancing ischaemic and bleeding risks for individual patients when selecting the type, dose and intensity of antithrombotic therapies (Table 1).