Endovascular stent grafting, or endovascular aneurysm repair (EVAR), is a newer form of treatment for abdominal aortic aneurysm that is less invasive than open surgery. Endovascular stent grafting uses an endovascular stent graft to reinforce the wall of the aorta and to help keep the damaged area from rupturing. The chimney/periscope technique (CH-EVAR) has been used to address complex aortic pathologies. This case report shows the outcomes of the endovascular management of an acute and simultaneous thrombosis of both renal stent grafts used in the endovascular repair of a complex abdominal aortic aneurysm (AAA).
Case Report
A 72-year-old man diagnosed with a 5.8 cm juxtarenal AAA was referred to our institution for consideration of complex endovascular repair. His medical history included active smoking, dyslipidaemia, hypertension and impaired renal function without a need for dialysis. He had been on various anti-hypertensives and statins without antiplatelet or anticoagulant therapy.
The option of a complex endovascular repair was offered to the patient, due to his age and non-suitable anatomy for a standard infrarenal endovascular aneurysm repair (EVAR). He had refused open repair and showed a preference for an endovascular repair with an expected shorter length of stay and avoidance of other morbidities associated with surgery and general anaesthesia.
The chimney technique has the potential to create a new sealing zone enough to treat patients with EVAR. The concept is to extend the sealing zone of a complex aneurysm neck, achieving the final goal of EVAR, i.e. the exclusion of the aneurysmal sac with the protection of essential aortic branches in the area of exclusion.
In this case, CH-EVAR technique was chosen because of the favourable anatomy of both renal arteries, angulation downward >20°, with a predictable, easy and fast cannulation from an upper vascular access in the left humeral artery; and also because our team has experience with this technique. We considered the alternative of a fenestrated customised device (F-EVAR) with two branches for renal arteries, but we usually reserve this technique for cases with access to three or more visceral vessels. The risk of endovascular device failure, the need for lifelong surveillance with EVAR and possible additional interventions in future were outlined to the patient, and he rejected the open surgery option.
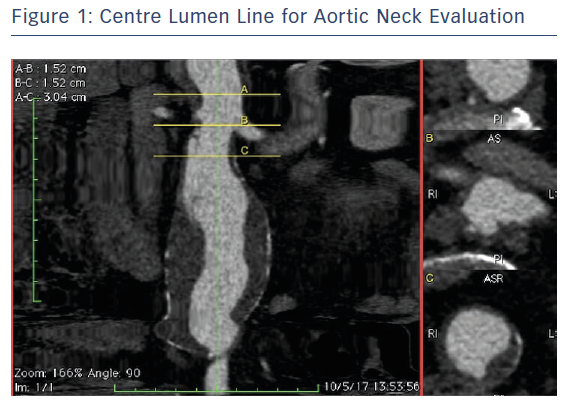
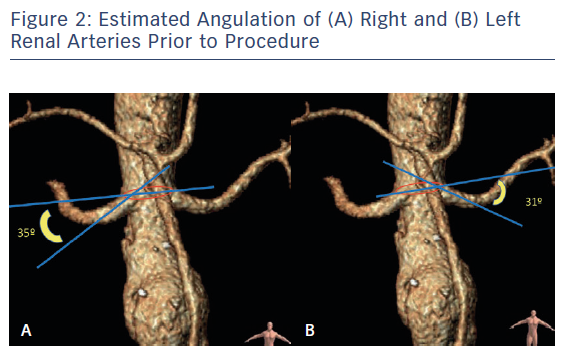
The AAA anatomy evaluation was performed using orthogonal multiplanar, centre lumen line and volume rendering reconstructions. The proximal neck was not good for infrarenal EVAR (reverse tapered neck, <0.5 cm length) and there was also a proximal shaggy thoracic aorta, which carries a risk of distal embolisation complications during the procedure (Figure 1). The diameter of the right renal artery 2 cm from the origin was 5.6 mm, and the diameter of the left at 2 cm was 5.7 mm. The downward angle was 31° on the left renal artery and 35° on the right renal artery. The renal branch angle was defined relative to the plane orthogonal to the aorta (Figure 2). Planning and sizing of the case was performed using OsiriX (Pixmeo) and Horos (Horos Project)software by two experienced EVAR vascular surgeons and reviewed with Medtronic experts with the use of 3mensio (Pie Medical Imaging) software.
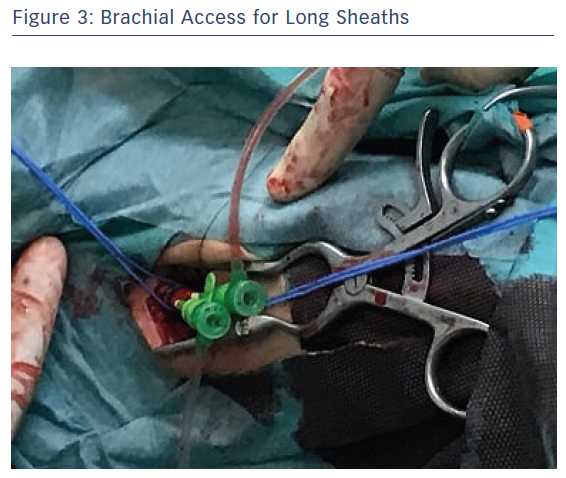
The procedure was performed under loco-regional anaesthesia with percutaneous bilateral ultrasound-guided femoral access, using 18 Fr right sheath and 14 Fr left sheath (GORE, Gore Medical), and open left humeral brachial access for the introduction of two 6 Fr long (65 mm) sheaths (Flexor, Cook Medical; Figure 3).
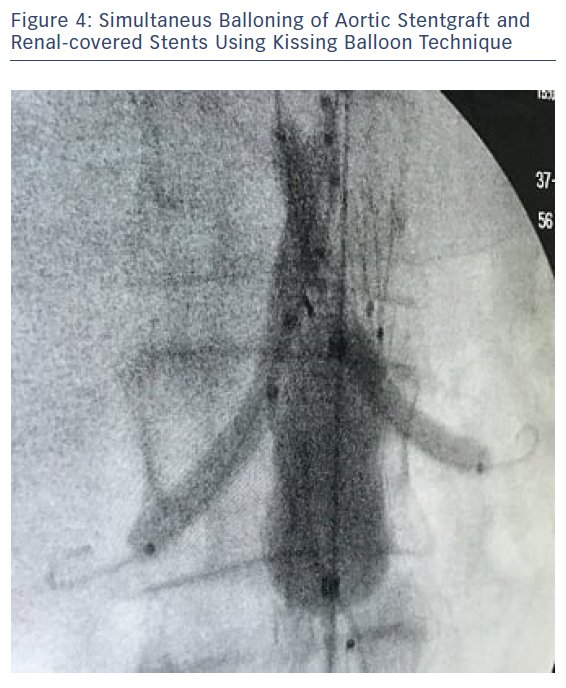
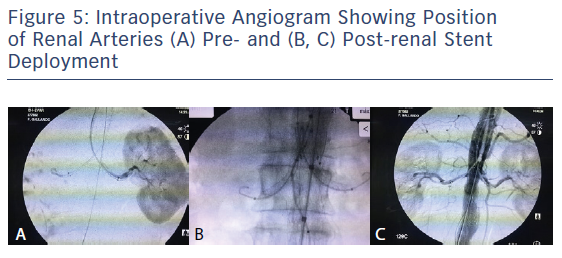
An oversizing of 30 % of the endograft is required, and thus our choice was the Endurant II bifurcated stent graft (ETBF 28-13c 124 EE; Medtronic) at the aortic neck and two 6 × 47 mm covered stent grafts (BeGraft, Bentley) for both renal arteries. Contralateral limb stent graft (ETLW 16-10c 82 EE; Medtronic) was deployed through the left femoral access. Simultaneous ballooning of the Reliant balloon (Medtronic) and the two covered stent grafts was undertaken using a 'kissing balloon technique' (Figure 4). Control angiogram showed no endoleaks, patency of both renal arteries, and no signs of renal dissection or stent grafts kinking, but with changes in both renal angulations before and post procedure (Figure 5). Operation time was about 130 minutes, and 300 ml of half-strength contrast was used, along with 5,000 IU heparin and 30 IU protamine at the end of the procedure. Closure was achieved using the Abbott Perclose Proglide suture mediated closure system.
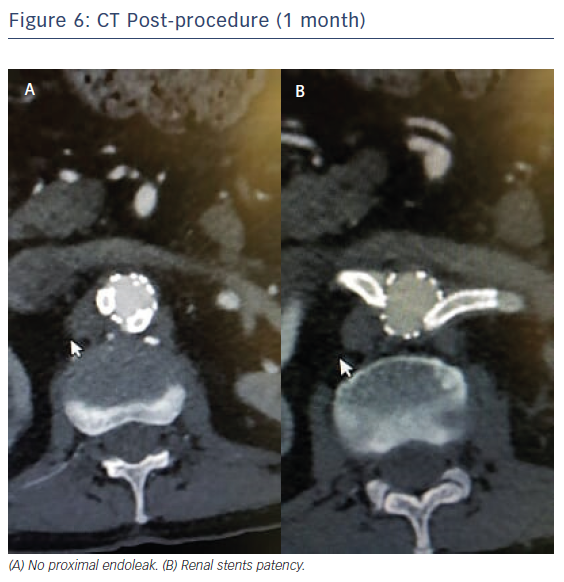
The patient was discharged 4 days after the procedure, a longer stay due to a 48 hour fever associated with post-implantation syndrome, with long-term antiplatelet therapy (clopidogrel) recommended. Follow-up at 1 and 3 months with CT and ultrasound showed no endoleaks, aneurysms shrinking (diameter <5.5 cm), and both renal stent grafts were patent (Figure 6). Renal angulation downward was 46° left and 44° right after implantation relative to the plane orthogonal to the aorta stent graft.
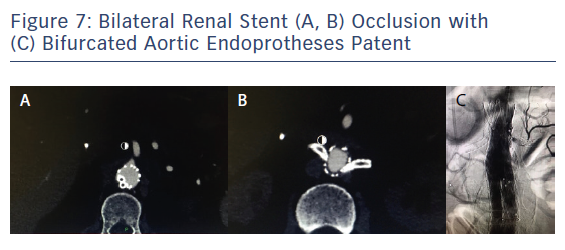
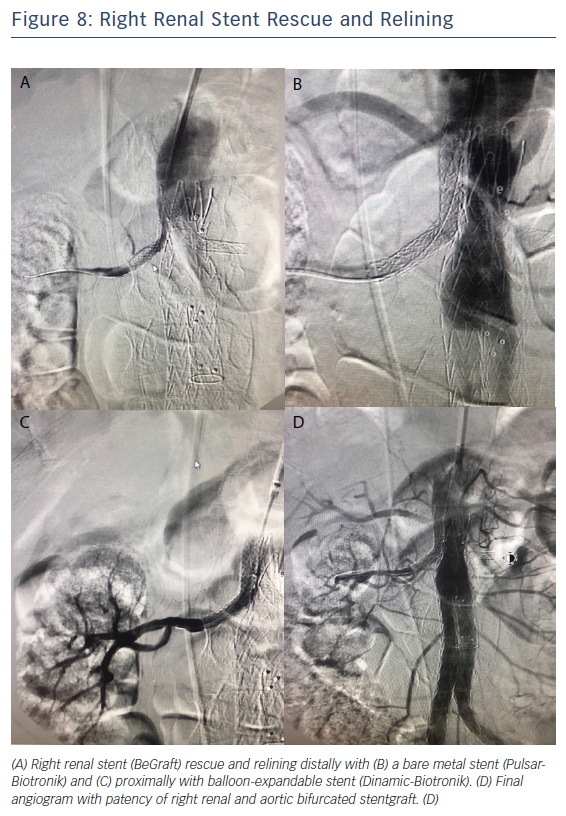
Four months after CH-EVAR, the patient was admitted to the emergency department of another centre. He had acute renal failure, with an emergent thoracic angio-CT showing aortic device patency but with changes at the suprarenal aorta thrombus and bilateral renal stent grafts occlusion, and a very poor distal right artery perfusion (Figure 7). He had haemodynamic instability and an emergent procedure was performed to try to rescue both renal arteries. Using a percutaneous approach from the left humeral artery, the right renal stent graft was successfully recanalised with a 0.035" hydrophilic Terumo guidewire after various attempts. A distal angiogram showed an acceptable distal perfusion of the right kidney with proximal total thrombosis of the stent. After intraluminal right renal covered stent recanalisation angioplasty with a 6 × 40 balloon was performed, observing a focal dissection just distal to the covered stent and also an area of possible kink at the medial part of the covered stent. A 7 × 60 Pulsar (Biotronik) self-expanding stent and a 7 × 38 Dynamic (Biotronik) balloon expandable stent were deployed for distal and proximal relining and correction of the previous stent kink. Recovery of the renal artery patency was immediately observed (Figure 8). Recovery of the left renal artery was not considered, due to the absence of any stump at the origin of the left renal chimney, the critical clinical situation of the patient and the long duration of the procedure (>180 minutes).
After the procedure, patient received haemofiltration for 1 week in the critical care department. His renal function recovered to previous levels and he was discharged at 15 days without need of haemodialysis. Recent 12-month follow-up shows right renal artery patency, no endoleaks and no signs of stent failure. The patient has fully recovered his normal activity, but is still a smoker.
Discussion
Juxtarenal aortic aneurysms are challenging cases for open surgery and there are still some controversies about adopting an endovascular approach as the first line of treatment. According to several articles in the literature, open surgery has remained the gold standard treatment for aortic pathologies, although endovascular techniques in the treatment of complex aortic pathologies have progressed greatly in recent times and are becoming a promising alternative in world reference centres.1-3
It is predicted that the number of endovascular repair procedures continues to advance, based on patients’ preference for non-invasive procedures and the increasing numbers of eligible patients with anatomically complex AAAs as juxtarenals for EVAR. Well-informed patients tend to refuse procedures with high morbidity and general anaesthesia, and will usually choose the option of a minimally invasive approach, even bearing in mind the risks of endovascular device failure and the need for subsequent interventions and lifelong surveillance with EVAR.
The chimney/periscope technique was introduced 15 years ago by Greenberg et al. as an adjunctive procedure to address challenging anatomic situations for which open surgical interventions presented high physiological risks.4 The PERICLES registry, published in 2015, has analysed the collected worldwide experience of snorkel/chimney EVAR for complex AAA. This is the largest series in the CH-EVAR literature and demonstrates comparable outcomes to those in published reports of branched/fenestrated devices, where primary patency reported was 94 %. These results have supported CH-EVAR as a valid off-the-shelf and immediately available alternative in the treatment of complex abdominal EVAR and provide impetus for the standardisation of these techniques.5 Many groups agree that chimney/periscope techniques could be used to tackle complex aortic pathologies.6,7 Reasons include no need for customisation compared with fenestrated grafts and better applicability in emergent cases; in addition these techniques can also be used in selected complex procedures, such as management of juxtarenal aortic aneurysms.6,7
An interesting recent review by Ultee et al. examined patients undergoing complex EVAR.8 They examined perioperative outcomes, focusing on differences with complex open repair, but also with standard infrarenal EVAR with patients undergoing non-ruptured complex repair in the American College of Surgeons National Surgical Quality Improvement Program. Their study included 4,584 patients, 9.0 % undergoing complex EVAR, 8.6 % undergoing complex open repair and 82.4 % undergoing infrarenal EVAR. Their results show a perioperative mortality of 3.4 % after complex EVAR, 6.6 % after open repair and only 1.5 % after infrarenal EVAR. Open repair was identified as an independent predictor of 30-day mortality, renal function deterioration and any complications. They concluded that for patients undergoing repair for anatomically complex AAAs, complex EVAR had fewer complications than complex open repair, but carried a higher risk of adverse outcomes than infrarenal EVAR. Over the last few years increasing numbers of reports in the literature have demonstrated promising short and mid-term outcomes and high rates of technical success (≥95 %) after CH-EVAR for complex aortic pathologies.8-10
The selection of devices for CH-EVAR has been evaluated with different types of aortic and renal stent grafts. In our case we chose the Endurant II stent graft combined with a covered balloon stent graft (BeGraft) because it has been reported in the PERICLES registry that the combination of nitinol/polyester stent graft devices with balloon expandable covered stent during chimney endovascular aneurysm repair is associated with improved survival compared with other aortic endografts.11
A variety of aortic stents grafts could be used as the main body, but only the Endurant II/IIs was CE mark approved for renal chimneys at the time of writing.12 The most frequently used stent design for the chimney technique is the covered balloon expandable stent, the use of the BeGraft monolayer balloon expandable stent graft provides some advantages, including a reduction in the size of the sheath and better conformability for vessel target. Despite the fact that all available stent designs (self-expanding, balloon expandable, non-covered and covered stents) have been applied in the chimney technique, at the present time the only on-label indication requires a covered balloon expandable stent.
Our patient shows no events at 3-month follow-up, with patency of both renal stents and no signs of thrombus, intimal hyperplasia or any kink or morphological failure at the stents on CT or ultrasound. Studies by Pecoraro et al. reported that the primary patencies at 12, 24 and 36 months were 94 %, 94 %, and 93 %, respectively, and other groups also have showed target vessel patency of 92 % and a secondary patency of 96 %, similar to those reported previously.6,13
The acute renal failure after 4 months was probably the result of occlusion of both renal stents. Usai et al. reviewed the literature on pararenal EVAR to determine the frequency and clinical relevance of chimney graft occlusion.14 They identified 83 studies regarding chimneys/snorkels for pararenal pathologies. The mean time to chimney graft occlusion reported was 3.5 months, so the renal stent occlusions in our case is consistent with these results.14 Although most groups identify a similar low rate of chimney graft occlusions, it generally happens a few months after stent placement. In many cases, the involvement of the renal artery had no severe clinical consequences compared with occlusion of other visceral vessels as the superior mesenteric artery. However, as we saw in this case, when both renal arteries are affected it can lead to a critical and dramatic situation. More detailed information regarding occluded chimney grafts will be needed in future to help identify the causes.
Different treatment strategies to solve stent occlusion after CH-EVAR have been reported, including open conversion, haemodialysis, placement of bare metal stents, placement of covered stents or conservative treatment.13,14
Our patient suffered acute bilateral renal thrombosis 4 months after the procedure, causing an acute renal failure with severe consequences that required critical care management. However, CT scans at 1 and 3 months did not demonstrate any sign of misplacement or significant kinking of the renal stents, with aneurysm sealing, no endoleaks and the size shrinking from 5.8 cm to 5.3 cm. The latter complication suggests that possibly our initial choice of BeGraft as the covered stent for renal arteries or some other technical details during CH-EVAR could have led to changes in the anatomy of the renal arteries. This might explain the late bilateral thrombosis, versus the more improbable option that the stents occlusion could be caused by a very selective distal embolisation of thrombus from the shaggy suprarenal aorta.
Ullery et al. reported the impact of renal artery angulation after evaluating the imaging of 77 patients who underwent complex EVAR (24 fenestrated, 53 snorkel/chimney) and measuring the renal angulation on preoperative scans and post procedure.15 For patients undergoing fenestrated EVAR, mean renal artery angulation was lower (mean -28° ± 21°) from patients receiving snorkel/chimney grafts (mean −30° ± 19°). Renal artery cannulation during fenestrated EVAR was performed significantly faster in arteries with less downward (≥−30°) angulation, whereas cannulation in snorkel/chimneys was faster in arteries with greater downward (<−30°) angulation. It can be explained by the anatomic fact that downward angulation makes it easier for the brachial access that is usually performed for snorkel or chimneys procedure. However, their results also show that immediate renal function decline, procedural complications and postoperative issues were not associated with renal artery angulation. In our case, the angulation of both renal arteries was measured using a similar method to Ullery et al. We measured the difference between an orthogonal centre line axis inside the aorta and the renal arteries axis, with both renal arteries with a significant downward angulation (−35° the right and −31° the left). Also, in our case, the renal cannulation was performed quickly (<15 minutes) and without any incidents.
The same group has also recently published other papers focused on dynamic geometric analysis of the anatomy of the renal arteries, quantifying the renal arteries geometry using a 3D model based on centre line extraction and computing the stent length, the branch angle, the end stent angle and the peak curvature within the stent.16 Although limited by having less experience, we agree with their initial assumption and paper conclusions about the fact that durability and patency of renal stent grafts after CH-EVAR may be related to how procedures and specific devices alter the native anatomy. The comparison of renal artery geometry in their review of 40 patients who underwent F-EVAR (n=21) or CH-EVAR (n=19) with a total of 72 renal artery stents shows that snorkels or chimneys had greater stented length compared to fenestrated renals, and this may be relevant as more artery covered more alteration of the original renal anatomy. With renal stent patencies of 97.2 % at mean follow-up of more than 1 year they report clinical incidence of acute renal failure occurred in 12.5 % of patients, although none required dialysis, but they do not report any case of bilateral occlusion. The final conclusions are that chimneys during EVAR induced significantly greater angle change at the stent end and curvature change distal to the stent compared to F-EVAR, although no difference in patency was noted. Chimneys exhibited greater end-stent angle changes compared to the F-EVAR.
After the re-evaluation of our case, we fit into their findings that these angle changes may exert differential effects on long-term renal artery patency, integrity and renal function following complex EVAR, and it could explain the bilateral thrombosis of the two renal arteries. In our case the original renal angulation downwards was remarkable and increased immediately after procedure, from 35° to 46° in the right and from 31° to 44° in the left, with a significant end-stent angulation increase, especially in the right renal.
The fact that chimneys for renal branches angled downward opposite to F-renal branches that angled upward has been also analysed with statistically significant results in 29 patients, with chimneys renals exhibiting an increased end-stent angulation (12±15°).17 A left renal snorkel stent thrombosis due to severe kinking, observed on 4-month postoperative imaging, is reported in this paper. The authors concluded that only chimneys resulted in significant end-stent angulation increase and suggests that these anatomic alterations are primarily generated early as a consequence of the procedure itself and, although persistent, they show no evidence of continued significant change during the follow-up.
Conclusion
To the authors’ knowledge, this is the first reported case of bilateral acute thrombosis of renal arteries after CH-EVAR solved with endovascular rescue of just one of the covered stents using a bare metal stent. Geometrical analysis shows how the combination of aortic stent grafts and covered renal stent grafts when performing chimneys procedures for complex endovascular repair induces changes in renal artery anatomy and how it could lead to and explain the early stent thrombosis. While the impact of these changes on stent graft durability and long-term patency has yet to be elucidated, such findings would support a theoretical caution for using chimneys with some specific covered stent grafts in extremely downward angulated renal arteries, even when it is a trend to select chimneys procedures with a homemade solution in these cases, due to the favourable position for cannulation of both renal arteries.
Direct renal access, angioplasty and relining of the previous stent with self-expanding and balloon expandable stents seems to be a suitable procedure to rescue an acute stent thrombosis, also reducing the distal end-stent angulation that could lead to more complications in the future.
Long-term complications including re-intervention rates after CH-EVAR are unknown and surveillance remains highly relevant. Future studies are warranted to further refine patient and anatomic selection for complex EVAR strategies and the application of geometric quantification techniques to longer term imaging studies would help to understand vessel anatomy’s changes following complex EVAR using different devices.