Atherosclerosis of the common femoral artery (CFA) is a common cause of lifestyle-limiting claudication and, less commonly, a cause of critical limb ischaemia (CLI). Located in the femoral triangle, the CFA is the major artery supplying blood to the thigh. Prior to the inguinal ligament, the external iliac artery provides in-line flow to the CFA. After crossing the inguinal ligament, the external iliac artery becomes the CFA, which then branches into the profunda and superficial femoral artery (SFA). Surgical CFA endarterectomy (CFE), with or without patch angioplasty, has been considered the gold standard for revascularisation, based on excellent procedural success and outstanding long-term patency.
Recent advances in endovascular therapies have made peripheral vascular interventions (PVI) more common for the treatment of peripheral artery disease (PAD), with the hope of reducing the need for surgical repair in selected patients. PVI is an attractive option for CFA stenosis for several reasons: PVI typically obviates the need for general anaesthesia, PVI is associated with fewer periprocedural complications and PVI requires shorter hospital stays.1–3 However, early studies of CFA PVI demonstrated inferior outcomes relative to CFE. CFA PVI endovascular procedures were plagued by early restenosis, reocclusion, and poor long-term outcomes.4 Despite these early setbacks, CFA PVI has become more common in recent years, owing to more options for endovascular therapies, operator familiarity, and an overall increased prevalence of PAD.2,5
CFA PVI with balloon angioplasty (BA), with or without adjunctive atherectomy and stent implantation, has been the subject of numerous studies. Given the emerging data in this field, the goal of this article is to summarise the current state-of-the-art techniques and their outcomes in the endovascular treatment of CFA atherosclerotic disease.
Surgical Endarterectomy
As a result of its superficial location, the CFA is easily accessible for surgery. A CFE can usually be completed in under a few hours, with an average hospital stay of 3–4 days.6 CFE can also be performed under local or general anaesthesia, although general anaesthesia is usually the preferred method to minimise patient discomfort.7 High rates of procedural success have been reported in the literature, approaching 95–100 %.6,7–10 CFE provides durable patency of the CFA, with a reported 5-year patency rate of 60–100 %.6–8,10
Although CFE is frequently performed, data are sparse on CFE outcomes. A periprocedural complication rate of approximately 6–10 % has been reported in published literature.6,11 Moreover, these complications can necessitate a repeat operation during the same hospital stay in up to 10 % of patients.6 The most common complications include infection, wound dehiscence and venous thromboembolism. However, more serious complications have also been noted including MI, cardiopulmonary arrest and stroke.11 In addition to these periprocedural complications, a 30-day mortality of 1.5 % has been reported.11 Careful patient selection is required to maximise the benefit of the procedure while minimising postprocedural complications.
Balloon Angioplasty
Given the relatively high rates of infection and need for reoperation with CFE, an endovascular approach may be preferred for certain patient subgroups, especially patients with multiple comorbidities. However, the first reports of CFA PVI were discouraging. Early devices were ill-suited to the complex atherosclerotic plaque that is often present in CFA disease. In a study of 984 patients treated with percutaneous transluminal angioplasty, acute and long-term restenosis was common, with overall reported 1-month, 1-year, and 3-year success rates of 77.9 %, 58.5 % and 36.6 % in CFA stenosis.4 Patients with diabetes and those with more complex lesions, including multi-vessel PAD and occluded lesions, were reported to have even lower rates of success. In addition, stent placement in the CFA has historically been avoided because of concerns about fracture and difficult endovascular access at the site of stent placement, despite some observations that patency is improved with stent placement.30 Stenting of the CFA has generally been reserved as a bail-out intervention for complications including recoil, acute restenosis or dissection.12,13 These observations, along with guideline recommendations that cemented CFE as the gold standard, initially resulted in only a small minority of CFA endovascular interventions.14 However, in recent years, device innovations and operator expertise have sparked numerous investigations into CFA PVI.
Studies examining CFA PVI have many limitations. CFA PVI investigations are often retrospective with relatively few patients included, indeed the largest cohort in CFA PVI studies is 1,014 patients.15 Several potential sources of bias exist as well; patients who undergo endovascular CFA PVI are often poor surgical candidates, have declined surgery or were offered PVI over surgery. These patients may have more complex atherosclerotic disease with higher rates of vascular and procedural complications. These studies should be considered hypothesis-generating until higher quality randomised and controlled trials are performed. However, a growing body of evidence in recent years is beginning to demonstrate safety and efficacy in the endovascular treatment of CFA atherosclerosis.
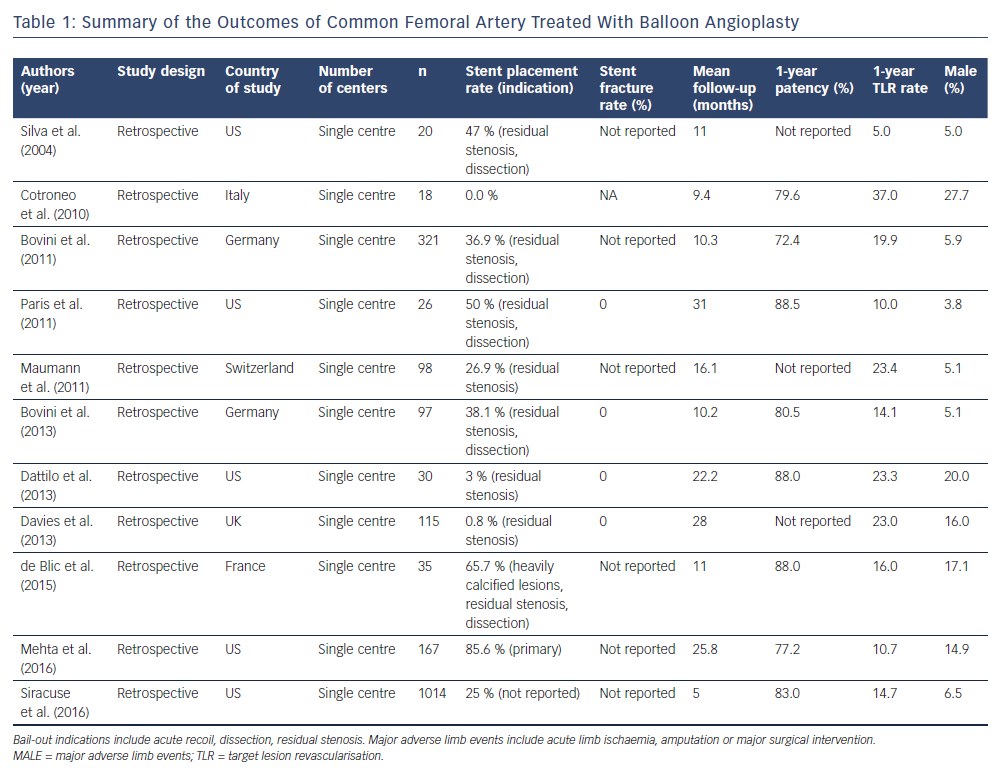
From 2004 to 2016, a total of 11 studies encompassing 1,990 patients treated with CFA PVI were identified in the literature. Table 1 summarises the key points of these studies. Major adverse limb events were defined as acute limb ischaemia, major amputation or major surgical intervention. Patients enrolled in these investigations ranged from poor surgical candidates to patients referred for PVI. These studies were analysed for procedural and patient-centred outcomes. The rates of claudication and CLI ranged from 44.4 % to 95.0 % and 5.0 % to 55.6 %, respectively. Procedural success (defined as <30 % residual stenosis) with BA was reported to be between 84 % and 100 %. Stent placement as a result of procedural complications ranged from 0 % to 50 %. Periprocedural complications including pseudoaneurysm, dissection, fistula formation, recoil and abrupt thrombosis occurred at a rate of 5.5 %–7.2 %, with most complications being self-limiting and requiring no additional interventions. However, up to 1 % of all patients required emergent surgery for bypass. One-year target lesion revascularisation (TLR) rates were reported to be between 5.0 % and 23.4 %, with up to 5.0 % of patients requiring surgical TLR.2,3,12,15–23 Longer-term outcomes are sparse, but three-year rates of freedom from TLR were reported at 57 %–86 %, with up to 12 % of patients requiring surgical intervention including bypass or amputation.3,24 Data on clinical improvement of claudication or CLI are sparse. As a surrogate measure, ankle–brachial indices (ABIs) increased between 0.23 and 0.30 points in selected studies at 1 year.12,16,19
Drug-coated Balloon Angioplasty
Because restenosis after standard BA is common, drug-coated balloons (DCB) may provide a means of increasing primary patency and freedom from TLR rates without the use of stents in CFA lesions.24 DCB treatment in the CFA provides an attractive alternative to conventional BA combined with stents. Two studies consisting of 56 patients have reported outcomes of DCB angioplasty to the CFA. The study population comprised patients with lifestyle-limiting claudication (53.8 %) and patients with CLI (46.2 %). At 1-year, TLR for all DCB treated lesions was 8.9%. Of those patients that required reintervention, surgical endarterectomy was performed in 3.5% of patients. Up to 12 % of patients treated with DCB required a major amputation. At 1-year, up to 55 % of patients treated with a DCB reported Rutherford category 1 symptoms. Bail-out stent placement was required in up to 10 % of patients.25,26
Most studies examined were single-centre, retrospective cohorts without randomisation or blinding, so there is a significant source of bias and self-selection. Although some of the reported data are encouraging, without high-quality clinical trials, these data should be interpreted as hypothesis-generating only. PVI of the CFA may be a viable alternative to surgery in the short term based on these studies but long-term data are lacking. PVI with conventional BA alone can offer a three-year TLR rate as low as 14.0 %, while DCB treated patients demonstrated a one-year TLR rate as low as 6.7 %; in both cases, TLR was most often a repeat endovascular procedure. Surgical revascularisation rates were reported to be up to 5 %. PVI periprocedural complication rates were significantly lower than with CFE, with most complications being self-limiting. More studies with larger cohorts and, ideally, randomisation or use of a pre-specified performance measure are needed to satisfactorily answer whether or not drug-coated balloon angioplasty of the CFA is an acceptable alternative to surgical endarterectomy.
Stent Implantation for Common Femoral Artery Disease
Concerns about vascular access, stent fracture, jailing of collateral vessels and acute stent thrombosis have all been raised as potential reasons to avoid stent implantation in the CFA. However, during PVI, the need for stents is sometimes unavoidable. As a result of suboptimal angiographic results, acute dissection, recoil or other complications of PVI, a stent usage rate of 36.9 % has been reported in a large single-centre study.3 Successes noted in the femoropopliteal vasculature, as well as a newer generation of stents engineered to prevent stent fracture, may change practice patterns regarding CFA stenting.27–29
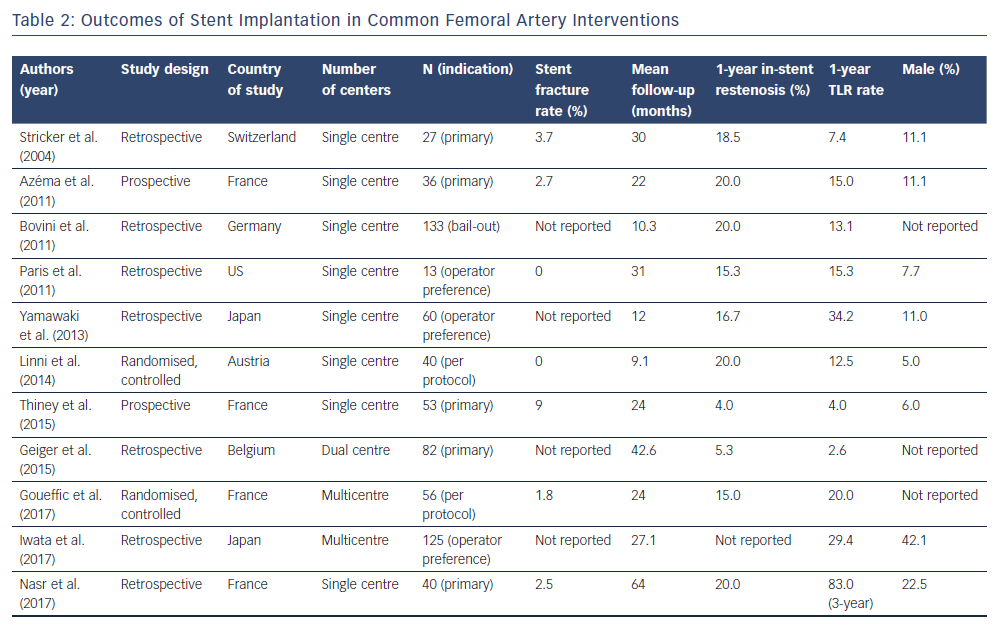
Stenting in the CFA is typically reserved for complications such as dissection, recoil or abrupt stenosis, so results must be viewed in the context of the indication for which stenting was employed. Universally, bare-metal stents have been studied. Studies examining a primary or secondary indication for stenting are summarised in Table 2.3,12,13,20,30–37 Only two randomised controlled trials of stenting in the CFA were found in the literature. These limitations should be considered when extrapolating outcomes from these patients.
The first study to objectively research stent implantation in the CFA was performed in 25 patients in 2004.30 In a retrospective case series, these patients were followed for a mean of 30 months with a primary endpoint of primary patency. The indication for peripheral intervention was claudication (82%). Most stents were self-expanding and only one balloon expandable stent was used. Procedural success was 100 %. No stent thrombosis was noted at 30 days. At 1- and 3-year follow-up cumulative patency was 86 % and 83 %, respectively. Stent fracture was only observed in one patient, in which a balloon expanded stent was used. This case series called into question whether the accepted practice of avoiding stenting in the CFA was warranted. The authors note that repeat PVI was required in two patients. Using fluoroscopy, the authors were able to access the stented vessel without damaging the stent. This suggests that stenting the CFA is both a safe and possibly viable method to maintain patency in the short and mid term.
Two randomised controlled trials have subsequently been performed in CFA PVI with stenting. In 2014, a study using bioabsorbable stents versus CFE was performed.33 This study is unique because it is the first study in which CFA PVI was compared against CFE directly and in which a bioabsorbable stent was used rather than conventional stents. A total of 80 patients were randomised to either CFE or CFA PVI with bioabsorbable stent. Technical success was achieved in 97.5 % of PVI procedures, with 2.0 % of patients requiring surgical revascularisation for acute occlusion and 17.5 % of CFE patients experiencing a minor surgical site infection. At 1 year, primary and secondary patency rates for CFA PVI were 80 % and 84 %, respectively, while CFE reported 100 % 1-year primary and secondary patency rates. CFA PVI patients had a limb salvage rate of 88 % versus 90 % for CFE patients. The 1-year TLR rate was reported at 2.5 % in the PVI group and 7.5 % for CFEs. Notably, 15 % of bioabsorbable stents had a complication of acute occlusion requiring additional endovascular or surgical intervention.
In 2017, a multicentre, prospective, randomised controlled trial of stenting in the CFA was performed.13 A total of 120 patients were randomised to either surgical revascularisation or PVI with stent placement. When possible, a self-expanding stent was placed. In the perioperative period 26.0 % of surgical patients had a complication; 63.0 % of these complications were delayed wound healing, although infection and haematoma were also noted. In contrast, the PVI group had a 12.5 % rate of complications. The rate of primary sustained clinical improvement (defined as claudication improved by 1 Rutherford class or resolution of chronic wounds and rest pain for CLI) was not significantly different between surgically revascularised patients and PVI patients; 76.1 % and 74.8 %, respectively. No significant difference was found in rates of TLR or primary patency at 24 months. At 24 months, only one stent fracture was identified, which did not require a repeat procedure.
Conventional stenting compared favourably with CFE, while bioabsorbable stents were associated with a lower rate of success and a higher rate of TLR. It has been hypothesised that bioabsorbable stents lack the radial force necessary for displacement of highly calcified lesions, thus impairing implantation into the vessel intima. A conventional stent fracture rate of 2 % was in line with prior investigations, suggesting the concern over stent fracture may be unfounded with newer generation nitinol stents. Patency rates at 1- and 2-year intervals compare favourably to published rates of CFE as well as CFA PVI without stenting. Although randomised controlled data exist for CFA stenting, more studies with larger cohorts are needed to truly settle the question of optimal CFA revascularisation.
Adjunctive Atherectomy
Atherosclerosis of the CFA is characterised by bulky, calcified plaques. Such plaques are less compliant, reducing the effectiveness of BA and increasing the risk of complications such as recoil and dissection. Debulking strategies including directional and orbital atherectomy, either as solitary therapy or combination therapy with BA and/or stenting, may provide more durable results than either therapy alone.
In the literature, two studies were identified that examined atherectomy use in CFA disease totalling 333 patients. Each study enrolled patients consecutively at a single centre who were analysed in a retrospective manner. In patients with claudication, when combined with BA, adjunctive atherectomy treatment resulted in significantly higher rates of primary patency at 20 months (92.3 % compared with 72.7 % in BA alone). In patients with CLI, 16-month primary patency rates were 78.4 % in combined BA and atherectomy, compared with 68.4 % in BA alone.21 When atherectomy was used as sole therapy it was associated with a patency rate of 87.1 % versus 66.7 % with BA alone at a 4-year interval.37 Stent placement was reported to be 0 % in BA with atherectomy.
Although current studies have a small sample size and may be subject to bias, atherectomy may provide an additional tool in PVI. Atherectomy has been used with good success in femoropopliteal disease, reducing the need for stent placement and improving TLR rates and patency outcomes.38,39 A tailored approached to CFA atherosclerosis involving debulking strategies when appropriate seems reasonable in the absence of high-quality clinical data that would otherwise guide decision making.
CFE remains the gold standard for the treatment of symptomatic CFA atherosclerosis. However, CFE is not without risk and requires careful patient selection to avoid surgical complications. CFA PVI may offer short- and mid-term patency rates comparable with CFE, but long-term data are lacking to help guide clinical decision making. Moreover, adjunctive therapies involving stents and atherectomy may offer additive benefits in maintaining patency and preventing the need for TLR. A dearth of high-quality clinical data offers opportunities for further avenues of research into the treatment of CFA atherosclerosis.