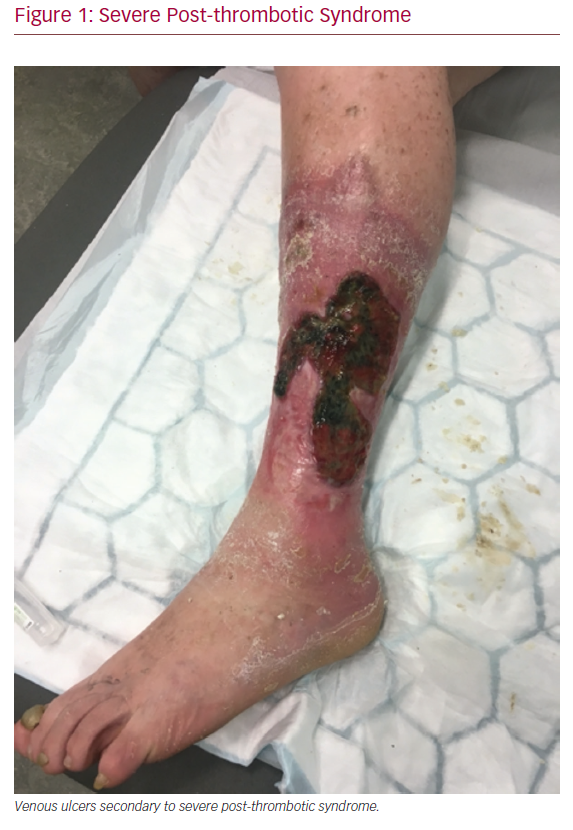
Deep vein thrombosis (DVT) is a common condition estimated to affect approximately 100,000 patients each year in the UK.1 DVT is a well-recognised cause of death through pulmonary embolism (PE), and, rarely, limb loss through phlegmasia cerulea dolens. Most commonly, however, DVT can lead to post-thrombotic syndrome (PTS), which affects patients of all ages, and is characterised by leg pain, itchiness, heaviness, swelling, skin discolouration, and, in severe cases, venous ulceration (Figure 1).2 Severe PTS has major socioeconomic consequences, and even mild PTS can have adverse effects on quality of life (QOL).3,4
Traditionally, anti-coagulation alone was used to prevent the propagation of DVT and PE and allow natural thrombus resolution. However, PTS is increasingly recognised as an important and common debilitating long-term sequela of DVT, given that failure of natural thrombus resolution can lead to a chronically occlusive post-thrombotic limb. PTS can occur in up to approximately 50% of patients in the 2 years after DVT, and is resistant to conservative and early thrombus removal therapies.5,6 Therefore, every effort should be made to reduce the risk of PTS when managing patients with DVT. The aim of this review is to summarise the pathophysiology and risk factors of PTS, highlight various risk reducing strategies for the development of PTS, and discuss future perspectives.
Diagnosis of Post-thrombotic Syndrome
PTS is the most common long-term complication after DVT. PTS is primarily a clinical diagnosis based on the presence of typical symptoms and signs of chronic venous hypertension in a patient with a previous DVT, but no objective diagnostic test exists.7 A number of diagnostic and severity scales have been developed for PTS: the Villalta scale, the Ginsberg measure, the Brandjes scale, the Widmer classification, the Clinical–Etiological–Anatomical–Pathological (CEAP) classification and the Venous Clinical Severity Score (VCSS).8 However, the Villalta scale has been validated externally and endorsed by scientific societies.9 On the Villalta scale, PTS is defined as a score ≥5, or a venous ulcer present, in a leg with previous DVT.10 The Villalta score classifies patients as having or not having PTS, and rates its severity, based on the sum of five venous symptoms and six clinical signs. Mild refers to a Villalta score of 5–9, moderate if the score is 10–14, and severe when the score is ≥15, or if a venous ulcer is present, regardless of the Villalta scoring parameters.10
Pathogenesis of Post-thrombotic Syndrome
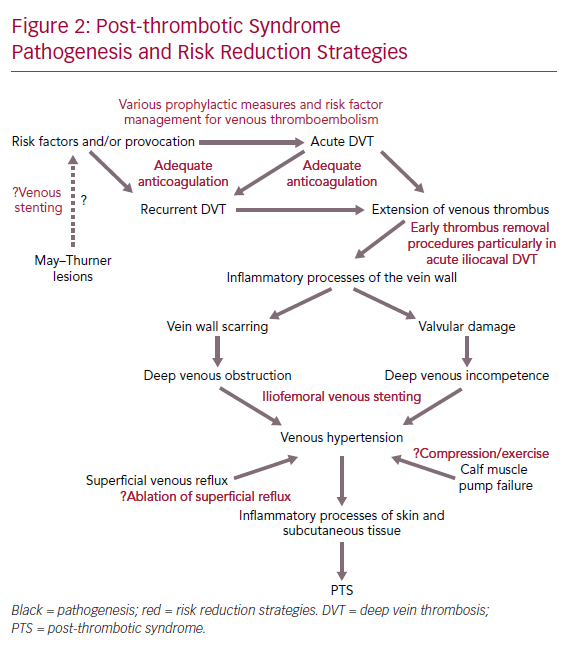
DVT can cause venous outflow obstruction and persistent reflux secondary to vein wall and valvular damage, leading to venous pooling, with limited reversibility depending on the location and extent of thrombus.11–14 This causes changes in microvasculature of the leg, leading to reduced perfusion of surrounding muscle, chronic inflammation, increased vascular permeability and scarring of the vessel wall.7 The presence of venous pooling indirectly affects distal deep veins and superficial collaterals causing dilation and incompetence. As a result, the calf muscle pump becomes ineffective and the ambulatory venous pressure fails to fall significantly with walking or exercise (as it does in the healthy state), which eventually leads to venous hypertension.15 Venous hypertension is thought to initiate chronic inflammatory cascades, which lead to features of PTS including venous claudication, ankle swelling, skin changes and even ulceration.16 However, PTS symptomatology varies over time.3 Figure 2 summarises the pathogenesis of PTS and potential risk reduction strategies.
Risk Factors for Post-thrombotic Syndrome
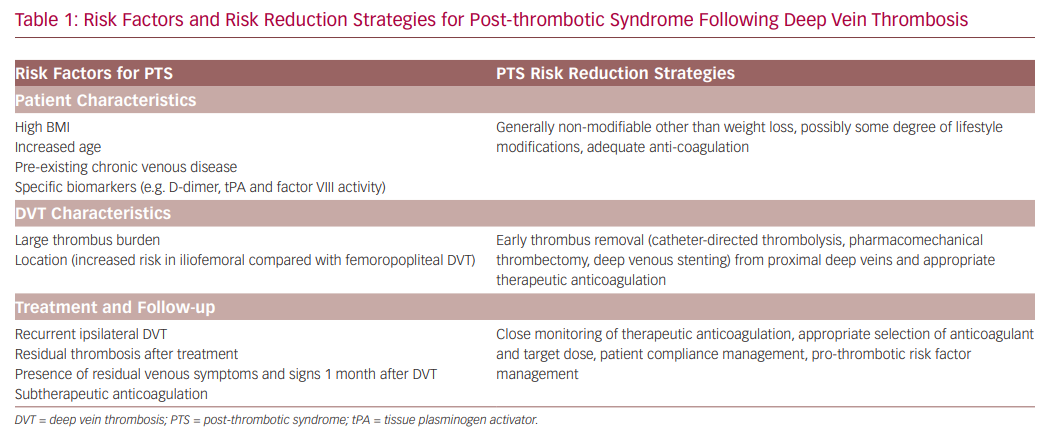
In clinical practice, it would be very useful to be able to predict the individual patient risk of developing PTS and its severity. As a result, prediction tools in the acute and sub-acute phase of DVT are being developed using baseline clinical and demographic characteristics.17–19 These baseline variables include age, BMI, sex, varicose veins, history of venous thrombosis, smoking status, provoked thrombosis and thrombus location. However, further validation is required before these risk scores are used in clinical practice. There are several key factors that increase the risk of developing PTS following DVT (Table 1).7,20–23 Identification of these risk factors, particularly modifiable ones, is important in strategic planning to minimise the risk of developing PTS; preventing DVT from occurring remains the most effective strategy.21 Therefore, it is important that individuals with increased risk of DVT are managed appropriately with the various prophylactic strategies available, such as anti-coagulation, compression hosiery, adequate mobilisation and lifestyle modifications. Strategies for the prevention of DVT are widely available in the literature, hence will not be covered in more detail in this review. Here, we focus on preventative and risk reduction strategies of PTS following DVT occurrence.
Preventative and Risk Reduction Strategies for Post-thrombotic Syndrome
Lifestyle Modification Strategies and Compression
Lifestyle Modifications
There are no studies to support any specific lifestyle modification that may prevent or reduce the risk of PTS. However, in patients with moderate or severe pain initially, early ambulation compared with bed rest was related to remission of acute pain in the affected limb.24 Regular exercise training in patients with PTS was also demonstrated to reduce severity of PTS symptoms and signs.25 Other lifestyle modifications that are likely to improve calf muscle pump, such as weight loss if obese, and frequent leg elevation, relieve some of the symptoms and signs of PTS, and improve wellbeing following DVT. Therefore, all patients with DVT should be counselled on these lifestyle modifications.
Compression
The graduated elastic compression stocking (GECS) has been central to PTS prevention for several decades and is thought to reduce both valvular reflux and venous hypertension.26–29 Although there have been several randomised controlled trials (RCTs) assessing the role of GECS in preventing PTS following DVT, all the studies were limited by their heterogeneity, including the interval between DVT diagnosis and compression, application, type, pressure, duration, co-intervention (in particular the type and duration of anticoagulation), first versus recurrent DVT, PTS diagnostic criteria, and length of follow-up.11,28–34 This heterogeneity complicates comparative analysis, but until recently, the overall evidence seemed to support GECS use for at least 2 years after DVT diagnosis to prevent future PTS.
This long-held belief in GECS has been challenged by evidence from the Compression Stockings to Prevent the Post-Thrombotic Syndrome (SOX) trial; a placebo-controlled, multicentre RCT that showed no benefit of GECS in preventing PTS.34 In the RCT, cumulative incidence of PTS was 14.2% with GECS compared with 12.7% in the placebo group. However, the trial was criticised for the low GECS compliance, with only 55.6% of patients wearing the GECS for ≥3 days per week at 2 years.
Two other studies with high GECS compliance demonstrated decreased PTS incidence, although it is unclear whether these studies included iliofemoral DVTs.30,31 This may suggest that GECS worn in a manner reflective of patient daily practice does not prevent PTS.35 As a result, there are some variations in the recommendation of the use of GECS following DVT among international and national guidelines. Overall, GECS may be used for symptom relief but the evidence of its role in preventing PTS is uncertain.36 In 2012, the National Institute for Health and Care Excellence (NICE) recommended offering below-knee GECS with ankle pressure >23 mmHg ≤3 weeks after the diagnosis of iliofemoral DVT. However, in 2015, following review of the SOX trial, NICE updated its guideline, and advised not to offer GECS following iliofemoral DVT for the prevention of PTS, but to use GECS only for symptomatic relief.37
More recently, the One versus Two Years of Elastic Compression Stockings for Prevention of Post-thrombotic Syndrome (OCTAVIA) study showed that stopping GECS after 1 year in patients with proximal DVT seemed to be non-inferior to continuing GECS for 2 years.38 In 2018, the Individualised versus Standard Duration of Elastic Compression Therapy for Prevention of Post-thrombotic Syndrome (IDEAL DVT) non-inferiority study showed that it was safe to shorten the duration of GECS on an individualised basis after proximal DVT for prevention of PTS.39 A further RCT, the multicentre Compression Hosiery to Avoid Post-thrombotic Syndrome (CHAPS) study (ISRCTN73041168) in the UK aims to address the effectiveness of GECS in preventing PTS in patients with DVT.40
Intermittent pneumatic compression (IPC) devices apply variable pressure cycles on the lower limb with inflatable compartments to emulate the calf muscle pump. Physiologically, IPC is thought to protect against venous thromboembolism (VTE) in a variety of ways: by reducing venous stasis, inducing flow-related venous endothelial alterations, improving lymphatic drainage and increasing endogenous fibrinolytic potential. A series of RCTs and meta-analyses have shown that IPC use alone reduces DVT incidence by more than 60%, with further reduction when concurrent pharmacological prophylaxis is used.41 These data have been supported by a 2016 Cochrane review that confirmed that, based on moderate quality evidence, IPC plus pharmacological prophylactic measures decreased PE incidence when compared with anticoagulation alone and decreased the incidence of DVT compared with GECS alone.42 Therefore, the usage of IPC to prevent postoperative DVT, particularly in high-risk cases, should be considered as part of a multi-modal PTS prevention strategy.
Medical Strategies
Anticoagulation
The use of anticoagulation after first acute DVT has the largest, proven benefit in reducing the incidence of PTS when compared with no treatment. Systemic anticoagulation therapy following DVT prevents the propagation of existing thrombi, formation of new DVT, PE, and recurrent DVT, all of which are known risk factors for the development of PTS.43 However, anticoagulation cannot lyse acute thrombus; this depends on the patient’s endogenous fibrinolytic system.44
Current practice following acute DVT is use of low-molecular-weight heparin followed by bridging to dose-adjusted oral vitamin K antagonists (VKA), such as warfarin, until an international normalised ratio (INR) target of 2–3 is achieved, at which point warfarin only is continued; or the use of a direct oral anticoagulant (DOAC) from day 1 with or without bridging parenteral therapy.45 The time in therapeutic range is critical to the effectiveness; for example, in warfarin therapy monitored using INR, a subtherapeutic anticoagulation (defined as INR <2 for >20% of the time) was associated with a significant increase in PTS development.46
Recently it was found that treatment of DVT with rivaroxaban might be associated with a lower risk for PTS development.47–49 In 2020, in a study of patients with acute proximal DVT, the risk of PTS in the DOAC-treated patients was reduced by 54% compared with patients treated with VKA (OR 0.46; 95% CI [0.33–0.63]).50 Although the authors advise interpreting the results with caution, they propose that patients treated with a DOAC, unlike those receiving VKAs, have progressively increased vein recanalisation over time. Overall, the anticoagulation strategy should be tailored to the individual, taking into account patient preference and compliance, comorbidities, polypharmacy, bleeding risk, DVT aetiology and risk of recurrence.51
Other Drugs
There is limited evidence to support venoactive drugs, such as rutosides (a herbal remedy used in chronic venous insufficiency to reduce swelling and skin changes), defibrotide (a single-stranded polydeoxyribonucleotide that has anti-thrombotic, anti-inflammatory and anti-ischaemic properties) and hidrosmin (a vasoprotective synthetic bioflavonoid) in preventing PTS following DVT, hence their use is not recommended at present.52,53 Sulodexide, a glycosaminoglycan consisting of unbranched polysaccharide chains with numerous biological effects including anti-thrombogenesis, anti-inflammatory effects, and endothelial protection, has recently been shown to potentially reduce recurrent VTE and PTS, although further clinical trials are needed to confirm its roles.54,55
Stategies for Early Thrombus Removal
There are two main indications for early removal of thrombus in patients with acute DVT. First, removal of thrombus may be needed in patients with severe pain and swelling, especially when there is increased risk of limb-threatening ischaemia such as phlegmasia cerulea dolens, or worsening symptoms despite optimal medical and conservative treatment. Second, early removal of thrombus in patients with iliofemoral DVT may reduce the risk of PTS development, as a result of the reduction in inflammation and injury to the vein wall and valves that would otherwise be caused by residual thrombus.
Several studies, including RCTs, reported that in selected patients, removal of acute thrombus resulted in better long-term outcomes compared with conservative measures alone in terms of reducing the risk of PTS.56–59 However, two recent multicentre RCTs have questioned the efficacy of early removal of thrombus to reduce PTS, although both studies were limited by various methodological flaws.6,60 Major guidance from NICE, and the American Venous Forum/Society for Vascular Surgery (AVF/SVS) recommend consideration of thrombus removal intervention in the 14 days after acute DVT; tissue plasminogen activator (tPA) has the greatest effect in experimental animal thrombi when fibrin content is greatest, between 7 and 10 days following induction.61
Surgical Thrombectomy
Until the 1970s, the principal method of removal of thrombus in acute DVT was surgical thrombectomy.62 Surgical thrombectomy did not show long-term benefits until it was combined with arteriovenous fistula formation distal to the site of venous reconstruction in order to improve venous inflow.63,64 However, this was suitable only for a select group of patients, and thrombosis recurred early if residual thrombus remained after the procedure. Surgical thrombectomy is not routinely performed, largely due to the invasiveness of the procedure, and to the significant potential for morbidity compared with percutaneous interventions. However, occasionally it is still indicated, particularly in patients with acute DVT requiring rapid removal of thrombus to relieve a limb-threatening ischaemia.
Systemic Thrombolysis
Systemic thrombolysis demonstrates superior clot lysis in acute DVT patients compared with conservative treatment alone.65 However, systemic thrombolysis (dose used varied; streptokinase the most common agent used, with and without heparin) was associated with a high rate of major bleeding complications such as intracranial haemorrhage and retroperitoneal haematoma.65 As such, systemic thrombolysis is not recommended in current practice.66 Furthermore, an early observation from Meyerovitz et al. showed that systemic treatment with thrombolytic agent did not permit sufficient penetration into occluded thrombi, a challenge that catheter-directed thrombolysis (CDT) and pharmacomechanical thrombolysis (PMT) were developed to overcome.67
Catheter-directed Thrombolysis
CDT was developed as a minimally invasive procedure with the aim of removing the bulk of the thrombus, leaving an ‘open vein’ with no obstruction to venous flow.68,69 In doing so, CDT has overcome many of the limitations of a systemic agent.70 The procedure involves ultrasound identification of a suitable vein for access (typically either popliteal, femoral or, more rarely, internal jugular vein) followed by introduction of a catheter into the deep venous system. This allows for targeted delivery of high concentrations of a fibrinolytic agent, such as tPA, directly into the occlusion site via a multi-sidehole catheter.
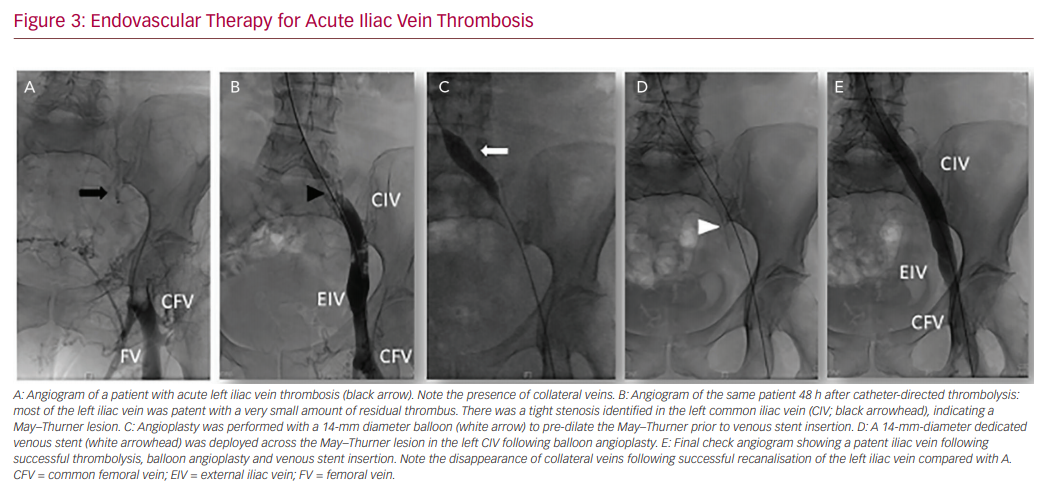
In our practice, a 10 mg bolus of alteplase is infused throughout the thrombus followed by 1 mg/h for 5 hours. A check venogram will generally be carried out after 12–24 h to assess the degree of thrombus dissolution, and evaluate the need for repeat thrombolysis and adjunctive angioplasty or stenting (Figure 3).71,72 When considering CDT, patient preference, as well as bleeding risk and comorbidities must be taken into account. Location and extent of DVT is important, given that isolated calf DVT has a much lower risk of PTS compared with iliocaval extension.28,73
Early studies demonstrated a reduction in incidence of PTS following CDT.56,74,75 The Catheter-directed Venous Thrombolysis in Acute Iliofemoral Vein Thrombosis (CaVenT) study, a multicentre RCT of 209 patients comparing CDT with standard treatment alone (anti-coagulation and compression), found significantly improved iliofemoral patency rates (65.9%) in patients treated with CDT (65.9% versus 47.4%, p=0.012).76 Additionally, after 2 and 5 years, there was a significant absolute risk reduction of 14.4% (41.1% in CDT versus 55.6% in control, 95% CI [0.2–27.9]; number needed to treat of 7, 95% CI [4–502]), and 28% (43% in CDT versus 71% in control, 95% CI [14–42]; number needed to treat of 4, 95% CI [2–7]), respectively. Interestingly, QOL at 5 years as measured with the EuroQol-5 Dimension (EQ-5D) and the disease-specific VEnous INsufficiency Epidemiological and Economic Study (VEINES)–Quality of Life/Symptoms (VEINES-QOL/Sym) questionnaires did not differ between treatment groups.77
CDT is recommended in the NICE and AVF/SVS guidelines for treatment of symptomatic iliofemoral DVT <14 days old, in patients with good functional capacity, life expectancy of more than 1 year and low risk of bleeding. However, potential limitations of CDT include time delay to lysis and hospital stay in a high dependency unit, with the associated economic implications, although this needs further research.66,78–80
Mechanical and Aspiration Thrombectomy
Mechanical and aspiration thrombectomy provides an alternate minimally invasive method of thrombus removal in the case of contraindications to pharmacological thrombolysis. Potential contraindications to pharmacological thrombolysis, such as CDT, include recent major surgery, trauma, stroke, pregnancy and active or recent bleeding. As the name implies, this form of thrombectomy uses mechanical force generated through rotatory and rheolytic means to break up the thrombus into smaller segments that can often then be aspirated. For example, a modern derivative of a Fogarty balloon is inserted distal to the thrombus and then retrieved with the thrombus.81,82 The rate of PE following surgical thrombectomy is <1%, which is comparable to the incidence in conservatively treated patients.83
Pharmacomechanical Thrombolysis
Several types of pharmacomechanical catheter-directed therapy have been developed to improve the efficiency of thrombus clearance compared with CDT or mechanical thrombectomy alone. PMT consists of an endovascular device that is advanced into the thrombus, which performs a combination of maceration and/or aspiration to physically break down the thrombus. This increases the surface area of residual thrombus for both exogenous and endogenous thrombolytic processes, reducing both the dose of thrombolytic agent required and the duration of thrombolysis.84–86 Limited evidence thus far suggests PMT is safe and effective in reducing PTS.87
The Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-directed Thrombolysis (ATTRACT) trial set out to address the treatment effect of adding PMT (catheter mediated or device mediated, with or without venous stenting) compared with anticoagulation alone on the incidence of PTS, measured with the Villalta score, in patients with acute proximal DVT.6 In all, 692 patients with acute proximal DVT were randomised to receive either anticoagulation alone or anticoagulation plus PMT. The ATTRACT trial reported no significant between-group difference in the percentage of patients with PTS between 6 and 24 months (48% versus 47%, respectively, p=0.56). Importantly, a significant reduction in moderate-to-severe PTS in patients receiving early thrombus removal therapy was observed (18% in the PMT group versus 24% of those in the anticoagulation alone group, p=0.04). Furthermore, when PTS was continuously assessed at 6, 12, 18 and 24 months after treatment, symptom severity scores at each follow-up were significantly lower in patients who had received PMT compared with anticoagulation alone (p<0.01).6 This suggests that although PMT did not prevent the onset of PTS, it resulted in significant symptom improvement. However, optimal timing of the intervention from the onset of DVT remains unclear.
A further post-hoc analysis of ATTRACT patients with iliofemoral DVT examined the effect of PMT.88 Although PMT did not influence the occurrence of PTS, it significantly reduced early leg symptoms, and, over 24 months, reduced PTS severity scores, and the proportion of patients who developed moderate-or-severe PTS. However, a limitation of this analysis was the substantial loss to follow-up that was unbalanced between the treatment groups (more missed PTS assessments in the non-PMT arm), which influenced the study’s estimates of treatment effects.6 More recently, the Dutch Catheter Versus Anticoagulation Alone for Acute Primary (Ilio)Femoral DVT (CAVA) trial with 184 participants randomised to ultrasound-accelerated CDT versus standard care only (anticoagulation, knee-high ECS and early ambulation) reported no significant difference in 1 year rates of PTS between the two groups (29% versus 35%).60 However, that study carried several limitations including a relatively small proportion of iliofemoral DVT patients and a low procedural technical success rate.
In the AVF/SVS guidelines, early thrombus removal with PMT over CDT is recommended ≤14 days after acute iliofemoral DVT if resources and expertise allow, due to improved efficacy and the more favourable safety profile.66 According to recent NICE recommendations, percutaneous mechanical thrombectomy for acute DVT of the leg has well-recognised but infrequent complications; hence the procedure should be used only with special arrangements for clinical governance, consent, and audit or research.89
Adjuncts to Thrombus Removal Procedures
Endovenous Balloon Angioplasty and Deep Venous Stenting
This is a growth area as technologies are being developed to remove ‘old clot’ from patients with established PTS. Old clot, however, contains little residual thrombus but is replaced by fibrous tissue.90 Endovenous balloon angioplasty and stenting of deep venous (particularly common femoral vein, iliac vein, and inferior vena cava) residual obstructive lesions after early thrombus removal procedures such as CDT and PMT are increasingly favoured.65,90 Venogram and/or intravascular ultrasound (IVUS) is used to identify and measure the degree and extent of obstructive lesions prior to balloon angioplasty and stent insertion. Although endovenous balloon angioplasty and stenting may reduce the risk of recurrent DVT and PTS in some patients, there is as yet no high-quality evidence to support the routine use of such procedures. Further research is required to identify the group of patients who may benefit from such adjunct intervention, and the optimal time and degree of venous stenosis for endovenous balloon angioplasty and stenting, and its cost effectiveness.
Due to the nature of the obstructive fibrotic and compressive lesions of the vein wall, balloon angioplasty alone is not sufficient, hence insertion of self-expandable stent is required to maintain the intended lumen diameter. Until recently, only stents designed for arterial pathology were used. However, several dedicated venous stents are currently available. These dedicated venous stents are made from nitinol (a metal alloy of nickel and titanium), with a size (diameter and length), strength, flexibility, and resistance to thrombosis tailored to the venous system and pathology, which differ from their arterial counterparts.91 Patency rates of dedicated venous stents at 12 and 24 months are encouraging,92,93 but longer-term results are awaited.
The abovementioned ATTRACT study, and CAVA trial both include venoplasty and/or stenting at the discretion of the operator following PMT in their management protocols.60 In a small prospective study looking specifically at venous stenting after CDT in extrinsic compression of iliac vein (e.g. May–Thurner or Cockett’s syndrome), the acute phase patency rate was 92.3%, and the mid-term patency rate was 90%.94 These non-occlusive, non-thrombotic lesions are significantly easier to treat compared with their thrombotic occlusive counterparts. May–Thurner or Cockett’s syndrome is most often characterised by extrinsic compression of the left common iliac vein by the overlying right common iliac artery, but compression may occur at multiple sites and commonly affects the left lower limb.
Future Research and Perspectives
Despite PTS being common and causing much physical, social and work-related morbidity, there has been little research interest in this area until recently. More research is required to understand, prevent and manage established PTS. Improving our understanding of the pathogenesis and natural history of DVT and PTS, including at the molecular level, will help in the identification and modification of the risk factors involved in DVT and PTS. Various potential molecular markers including d-dimer, factor VIII, soluble thrombomodulin, tPA and specific genetic and inflammatory markers are currently being investigated for their prognostic value in PTS.22,23,95,96 Developing objective and validated risk stratifications may help identify high-risk patients who may benefit from more aggressive measures to reduce the risk of PTS. Early removal of thrombus is associated with reduction of PTS risk in patients with acute iliofemoral DVT through effective recanalisation of the venous system, and reduction of venous wall and valve injury. There is also evidence from RCTs to support the role of early CDT in reducing the rate of PTS in patients with acute iliofemoral DVT. Further consensus and guidance are also needed in postoperative anticoagulation strategies to maintain long-term stent patency. Advances in imaging technology have provided opportunities to develop modalities that are able to characterise the thrombus.
Saha et al. are currently researching the use of magnetic resonance in direct thrombus imaging in measuring the age of the thrombus, which may help with patient selection for endovenous therapies.61 Simple measures, including compression and regular exercise, still require further high-quality trials to clarify their roles in reducing the risk of PTS development. Advancements in endovascular technology, such as PMT and mechanical thrombectomy devices, and dedicated venous stents, have provided enormous research opportunities into the prevention and improved management of established PTS. Research on bioprosthetic venous valves is also potentially helpful in the prevention of PTS.