Cancer survival rates have increased threefold in the last few decades because of advances in diagnosis and treatment. Radiotherapy has been used in multidisciplinary cancer control plans for a long time.
The current radiotherapy protocols have significantly improved the prognosis of many cancers, with a consequent positive impact on patient longevity. Regrettably, this also means that the latent effects of radiation now have more chance to present themselves, with a corresponding decrease in quality of life.1–3
Radiation sickness or acute radiation syndrome (ARS) occurs due to a brief period of high-dose ionising radiation. ARS involves reactive oxygen species-mediated DNA damage, which manifests – in increasing severity, according to the dosage – as mucositis, bone marrow suppression, endothelial cell damage and neurological effects. ARS occurs at radiation rates higher than 0.1 Gy/h, may last for months and can be fatal. This acute phase occurs in 60–80% of patients treated with abdominal or pelvic radiotherapy and is considered a risk of treatment intolerance for which a modification may be necessary.4–6
Chronic radiation syndrome (CRS) can be defined as the whole body’s systemic response to chronic total body exposure. In its initial phase, it is considered as a ‘dysregulatory pathology’ because of the involvement of the regulatory systems. The exact mechanism of development of late radiation effects is only partially understood and vascular changes in the form of endarteritis obliterans and telangiectasia are usually seen. Occasionally, ARS can be very severe and there will be no resolution; and in such cases the radiation injuries become chronic and indistinguishable from the delayed features, called ‘consequential effects’. CRS has been identified in survivors of Hiroshima and Nagasaki, Kyshtym, Chernobyl and the Fukushima nuclear disasters.
Akleyev has described the manifestations of CRS and defined the latent period to be 1–5 years. The CRS formation period coincides in time with the exposure at the highest dose rate. The symptoms are non-specific and usually involve multiple organs, particularly with regard to haematopoiesis and the nervous system. The recovery period usually starts 3–12 months after the termination of exposure or following a considerable reduction in the exposure rate. Haematopoietic impact can be fully reversible, as can the functional neurologic impairment, but ostealgic syndrome and micro-organic disorders usually last longer. The period of late effects may follow the recovery period.4,5,7
In DNA-damaged cells, sometimes the damage can be detected and repaired, but if not then apoptosis may be triggered. Alternatively, in the case of non-lethal DNA defects (i.e. novel mutations), subsequent divisions will pass these mutations on to the whole line and may predispose to carcinogenesis or teratogenesis. Secondary malignancies can occur a few decades after radiotherapy, within the irradiation field as well as remote from it.8
Apart from the increased risk for carcinogenesis, radiation-induced cardiovascular disease (RICVD) is a well-known sequel to radiation exposure. RICVD occurs both in patients with low cardiovascular risk and healthy vascular beds, and those with established atherosclerotic cardiovascular disease. It accelerates the process of atherogenesis. Both acute and chronic RICVD can present as pericarditis. In addition, both coronary artery disease and peripheral vascular disease may complicate the radiation exposure and present after many years.9
Chronic cellular exposure to elevated reactive oxygen species and sustained nuclear factor kappa B activation result in a chronic inflammatory state with the subsequent ineffective healing and remodelling. Additionally, vascular endothelial growth factor depletion will lead to disturbed angiogenesis. Impaired relaxation due to humoral and mechanical factors is another contributing cause that will lead to turbulence formation and, consequently, accentuation of the atherosclerotic process.10,11
Pathology of Radiation Arteritis
The correlation between the pathological findings and radiation exposure was first noted by Gassman, a few years after the introduction of the X-ray.12
Morphologically, in the radiotherapy field, the diseased arterial segment is usually sharply demarcated, contracted and narrowed with a relative pallor compared with the unaffected segments. The intima appears diffusely thickened, faintly granular and roughened with delicate wrinkles. Multiple white fibrous minute plaques can be seen in the intima, especially in the posterior walls, and most commonly longitudinally oriented. In some cases, these plaques coalesce to form larger ones. In contrast to atherosclerotic plaques, the yellowish pigmentation and fatty streaks are far less frequent. The adventitia is fibrous and indurated. The length of the diseased segment is variable and proportional to the radiation field. As a result, vessel wall thickening, lumen progressive narrowing and occlusion, pseudoaneurysm formation, vessel rupture, thrombus formation and distal embolisation can occur.13,14
Microscopically, circumferential alteration to the internal elastic lamina can be observed. Focal beading and fragmentation of elastic layers are also detectable. Moreover, loss of the refractile quality of elastic lamellae leads to a granular and swollen appearance. Regeneration of the disrupted membranes occurs with acid mucopolysaccharide accumulation, in addition to the proliferation of plump fibroblasts and collagen deposition; hence, intimal thickening (focal or diffuse), elevation and plaque formation. Variable plaque growth sequences are suggested as the plaque composition has a poor correlation with age. Injury to vasa vasora, ischaemic necrosis, hyalinisation and thickening of the vessel wall with fibrin deposition can also be seen as well.13,14
Broadly speaking, the radiation arteritis lesions may be categorised, according to the time elapsed since exposure, into the following: early lesions (up to 5 years), with a predominance of mural thrombosis; intermediate lesions (5–10 years), where panmural fibrosis, occlusion and the relative paucity of collateralisation can be seen; and late lesions (mean, 26 years), including periarterial fibrosis and atherosclerosis.15
Radiologically, the diagnosis can be made in the following cases: detection of the typical lesion in the radiotherapy field; when the typical lesion is of a long, uniform sub-occlusive nature in the involved vessel; and for other forms of lesion such as tight stenosis, multiple stenoses and subtotal or total occlusion.16–18
There can also be relative sparing of the arteries outside the irradiation field. They do not show radiologic abnormalities except in patients with well-known atherosclerotic disease.
Stenosis and thrombosis of the major abdominal and pelvic veins should always be looked after. Fibrosis and tight stenosis of the superior vena cava have been reported 5 years after the completion of bronchogenic carcinoma treatment and radiotherapy. Other non-vascular tissues may show radiation-related changes as well.
Clinical Considerations
Pelvic radiation disease (PRD) may lead to radiation-induced damage to the nearby non-cancerous tissues in the gastrointestinal, genitourinary, dermatological, haematological and musculoskeletal systems. All major pelvic vessels are susceptible to chronic inflammatory changes, which eventually lead to stiffness, stenoses, fibrosis and accelerated atherosclerosis.19–21 These changes are as follows:
- Microvascular changes, such as generalised capillary network failure, vasa vasora injury and vessel blockage by endothelial sloughing.
- Arteritis, which may present as acute or critical limb ischaemia or as worsening claudication.
- Radiation-associated venous stenosis of the iliac segment (with or without a history of venous thrombosis), which is a cause of chronic limb pain, swelling, discolouration or ulceration. Therefore, it is not uncommon to find multiple admissions to the accident and emergency department in the previous medical records of these patients, with a clinical picture suggestive of deep vein thrombosis (DVT) with negative venous duplex scans for DVT. In this condition, severe chronic venous insufficiency (CVI) due to radiation-associated iliac venous stenosis must be considered in the management plan.
- Lymphovascular fibrosis with a proximal obliteration pattern lymphoedema.
- Mixed involvements.
In addition, the management process of radiation-induced peripheral vascular disease (RIPVD) is usually challenging in both diagnosis and treatment, due to the following considerations:2,9,14,19,21–26
- RIPVD is sometimes diagnosed a decade or more after radiation; this delayed occurrence makes it less likely to be considered as a diagnostic possibility. The complex nature of PRD and RIPVD, with multiple system involvements and wide-ranging symptoms, makes the diagnosis more challenging. Similarly, the multifactorial limb swelling and/or lymphoedema may add to the diagnostic and therapeutic challenges.
- The difficulty of clinical examination and lower limb arterial pulse detection, due to skin induration and hardening, CVI, chronic limb swelling and lymphoedema.
- Presentation can be altered by the accompanying lumbosacral radiculo-plexopathy, which is a possible consequence of radiotherapy, which may be manifested by a varying degrees of motor and sensory impairments.
- There may be vasculitis and leucocytoclastic symptoms, due to chemotherapeutic agents, such as oxaliplatin. These may present as digital gangrene, livedoid reticulopathy and purpura. Clearly, the aggregation of the large vessel and small vessel disease features is more demanding in terms of making the appropriate diagnosis and planning the treatment strategy, with a less promising prognosis.
- There may be adjuvant and/or neoadjuvant cancer immunotherapy with angiogenesis inhibitors, such as bevacizumab and ponatinib, which are well-known causes of atherosclerosis and cardiovascular disease. Hypertension, ischaemic heart disease, aortic dissection and involvement of large- and medium-sized vessels are all reported in treated patients.
- Cancer recurrence and the associated paraneoplastic syndromes that accompany certain types of cancers may present with vaso-occlusive digital and small vessel disease patterns. In this setting, it is exceedingly difficult to assign this very distal disease to a specific cause from this long list of possible aetiologies. This is particularly problematic if the cancer recurrence has not yet been diagnosed.
- There may be unduly delayed development of collateral circulation when compared with the classical atherosclerotic peripheral vascular disease, possibly due to radiation effects on the whole radiation field tissues, generalised capillary failure and extensive fibrosis, leading to faster progression of foot necrosis. The heralding phase of intermittent claudication and rest pain may be very faint clinically and its duration is usually much shorter than in the standard atherosclerotic disease, with a narrower window of time to prevent major tissue loss. With consideration of the previously mentioned factors, the distal foot circulation deterioration may be rapidly evolving and overwhelming.
- Multiple diagnostic and therapeutic procedures associated with primary cancer, such as abdominal and pelvic cancer resection, stomas, inguinal lymph node biopsy and groin vascular accesses, all add to the difficulties of management and decision-making.
- The pathological changes to the irradiated vessels may take very long to be radiologically and clinically significant and this is an ongoing process taking place within the irradiation field. Therefore, it is not uncommon to see early failure and/or new lesions in the vicinity after the initial revascularisation procedure. Hence, it is vital to consider treating the whole pathological segment or bypass it altogether.
- The chronic inflammatory changes in almost all tissue types in the radiation field, from the skin (radiation dermatitis or ulcer) through to the bone (osteonecrosis), and the associated radiation enteritis and proctitis, add much to the complexity of any planned surgical approach.
- Management is complicated by the older age of a considerable percentage of patients who have had radiation therapy for pelvic malignancy, and the associated greater likelihood of multiple comorbidities, poor functional status and impaired immune response, as well as arteriopathy in different territories. Peripheral vascular disease in this cohort of patients may be multi-level in nature, which will complicate the process of revascularisation.
- Medical nephropathy and obstructive uropathy are possible comorbidities in people receiving radiotherapy. Exposure to chemotherapy, radiation-induced renal artery stenosis, ureteral strictures and/or compression may be causative factors.
- The rates of postoperative anastomotic and septic complications and redo bypass are much higher for radiation arteritis than for other indications.
Magnitude of the Problem
It must be emphasised that RIPVD – like any other radiation-related late sequel – is largely underestimated and the diagnosis can be missed very easily. It was once reported that the incidence of radiation enteritis is as common as Crohn’s ileitis.19 Information on RIPVD and iliac radiation arteritis incidence is lacking, but it is obvious that the incidence is increasing.9,19
Many cases of iliac radiation arteritis have been treated as standard peripheral vascular disease or as mixed arterial/venous leg ulcers. The history of previous radiotherapy to the pelvis may be totally missed or may not have been linked to the clinical situation. Consequently, unnecessary difficulties, unexpected operative challenges, and easily avoidable complications may be encountered as a result of the rush to explore the ‘radiotherapy groins’.16,17
More than 50% of patients with cancer receive radiotherapy. According to Bergonié and Tribondeau in 1906, a decade after the discovery of radiation, tissue radiosensitivity is directly proportional to the mitotic capability and potentials of proliferation, and inversely proportional to the degree of differentiation. Tissue radiosensitivity is stratified according to the Casarett or the Michalowski systems. Generally, genital glands, lymphatic, haemopoietic and foetal tissues are highly radiosensitive. Iliac radiation arteritis has been reported after radiotherapy for gynaecological (ovarian, cervical and endometrial) cancers, colorectal cancer, and lymphomas.27–30
In some reports, the diagnosis occurred 25 and 28 years after the radiotherapy, or even later.31 A very severe and rare form of radiation arteritis that involved the whole length of the infrarenal abdominal aorta, along with the visceral branches, as well as the bifurcation, was reported after the 31st birthday of a woman who had undergone nephrectomy and radiotherapy due to Wilms’ tumour at the age
of 5 years.32
Cardiovascular complications were also noted in more than 10% of Hodgkin’s lymphoma patients followed for a median of 9 years
after radiotherapy.33
The reported radiotherapy dose associated with arteritis is 20–80 Gy. Additionally, the specific radiation dose associated with iliofemoral radiation arthritis is 39.5–80 Gy. Both stenosis incidence and stenosis severity are proportional to the radiation dose and duration.17,34
Interestingly, colorectal cancer survival rates are higher in women, and there is no clear explanation. Moreover, the outcome of treatment for gynaecological malignancy is improving. Hence, it is not surprising that iliac radiation arteritis is more commonly diagnosed in women.35
Radiation Field and Delivery Methods
Iliac vessels are vulnerable to radiation effects because of the treatment of the nodal clinical targets rather than of the primary itself. The nodal clinical target volume has been defined in some protocols as the area in a 7 mm margin around the major vascular structures in the pelvis. It is subdivided into five groups: common iliac; external iliac; internal iliac; obturator; and presacral.
Planning CT has replaced X-ray markers as a prerequisite to proceed with radiotherapy. The new radiotherapy field protocols have been designed to reduce the damage to the surrounding tissues, but this reduction of radiotherapy dosage delivery to the surrounding pelvic tissues has increased the chance of missing the local pelvic microscopic disease.
In lymphomas, the radiotherapy dose varies according to the type, grade and stage of the disease, which can be as high as 40 Gy/20 fractions. Femoral, iliac and paraaortic nodes are potential targets.
Colorectal cancer treatment involves either short-course radiotherapy (SCRT) or long-course radiotherapy/chemoradiotherapy. SCRT is given at a dose of 5 Gy for 5 days or 1 week. Long-course radiotherapy/chemoradiotherapy patients are given either 2 Gy per fraction for 5 weeks or 1.8 Gy per fraction for 5.5 weeks.
Radiotherapy delivery is achieved by either of the following ways: anterior and posterior fields; or four-field box/brick, in which two lateral fields are also included.
In the management of cervical or uterine malignancy, treatment is given 5 days/week, 200 cGy/day, with all fields treated daily.
When managing urological malignancy such as prostate cancer, the organs at risk include bladder, rectum, intestine and femoral heads. The occurrence of radiation-induced iliac arteritis after radiotherapy for prostate cancer needs to be investigated, due to the lack of data currently available on this association.36–39
Decision-Making Regarding Treatment Options
Treatment of radiation arteritis of the iliac segment presenting with rest pain, tissue loss or acute limb ischaemia is broadly the same as for atherosclerotic/thrombo-embolic disease, although the challenges listed in the earlier section still need to be kept in mind.
The following principles are vital when considering revascularisation.
Urgent revascularisation is crucial, given that the clinical course and tissue loss are more dramatic when compared with the standard atherosclerotic disease. It is not uncommon for these patients to present with acute-on-chronic lower limb ischaemia.
Restoration of adequate perfusion can be accomplished with an endovascular or surgical approach.
The patient should be actively involved in the decision-making process after a clear and detailed discussion of the options and the challenges due to the nature of this disease, and this should include major amputation and mortality.
Angioplasty of the iliac arterial segment is the recommended first choice. It can be straightforward and produces satisfactory revascularisation. Nevertheless, the complications are much more frequent than with interventions for atherosclerotic lesions. Obviously, angioplasty can be the only feasible option in the case of bilateral iliac disease, because the bypass options are more complex in such circumstances. Treatment of symptomatic iliac vein disease is an integral part of the management. To the best of our knowledge, however, no reports have yet discussed the outcome of placement of concomitant ipsilateral iliac arterial and venous stenting. Thus, if venoplasty and stenting are deemed necessary, cross-over bypass seems a more logical option.
The complications are: detachment of an existing thrombus and distal embolisation; inadequate dilatation, persistence of a waist or failure to deal with the lesion because of the unduly stiff affected segment; dissection; perforation and rupture; and puncture-site bleeding, which is much more difficult to deal with.
Therefore: it is advisable to avoid the affected groin as an angioaccess; preferably, contralateral femoral, radial, brachial or ipsilateral midthigh superficial femoral access can be tried instead; thrombolysis has also been considered in some protocols as an integral part of the intervention; covered stents may be used primarily or at least kept on standby; completion angiogram is mandatory not only to ensure satisfactory dilatation of the lesion or to rule out extravasation, but also to exclude distal embolism at the popliteal trifurcation or pedal arch; and the operator needs to be ready to do surgery for unsuccessful or complicated intervention if required.
The simplest surgical revascularisation option is a short extra-anatomic bypass from the contralateral groin (common femoral artery; CFA) to the ipsilateral superficial femoral artery (SFA) in a healthy area just distal to the radiotherapy field effects. The advantages of this bypass are that it is technically less demanding; it is less time-consuming; the bypass is short; it has longer patency; and it totally excludes the affected groin.
Anastomotic and septic complications, however, are more frequent due to local and systemic factors. Other – more complex – surgical revascularisation options may be necessary for bilateral or multilevel disease. Moreover, in unsuccessful and complicated angioplasty cases, surgical treatment can be far more demanding and less promising.16,17,19,26,29
Four Cases of Iliac Radiation Arteritis
The following case reports are discussed in an attempt to highlight, as much as possible, the aetiological and clinical characteristics of iliac radiation arteritis.
Case 1
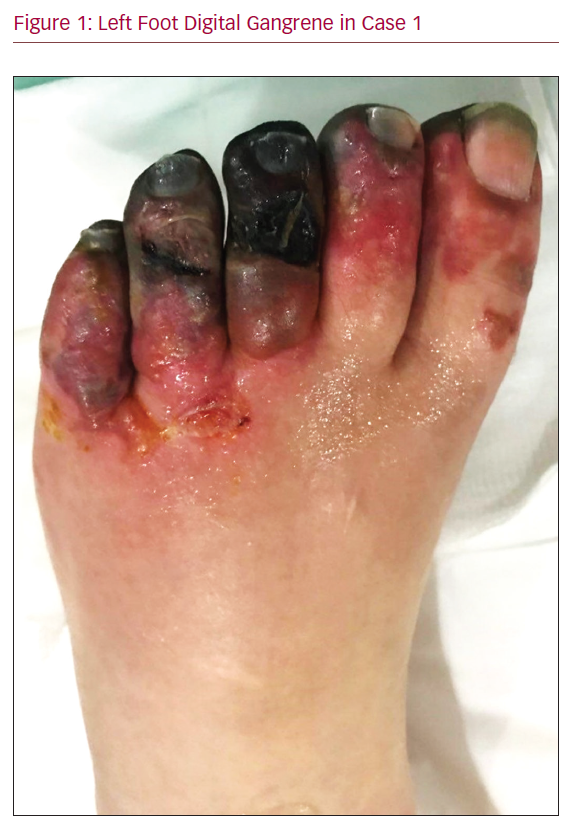
A 59-year-old female patient presented in 2011 with gangrene of all toes of the left foot, 14 years after pelvic irradiation for endometrial cancer. There was no clinical evidence of tumour recurrence and no other comorbidities or arteriopathy. The left foot was mildly swollen and all toes were gangrenous, with multiple blisters, mottled skin patches and minute ulcers (Figure 1). Ankle–brachial pressure index (ABPI) was 0.79 on the right side and 0.35 on the left side.
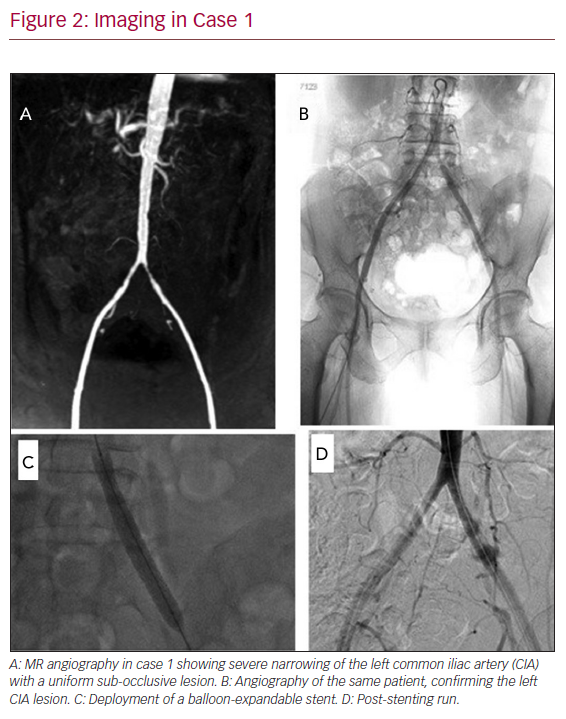
Magnetic resonance angiography indicated a left common iliac artery (CIA) 3 cm stenosis with a mild narrowing of the internal iliac artery (IIA) ostium, plus non-significant changes in the right CIA. No other arterial segments were involved (Figures 2A and 2B).
Endovascular treatment was suggested to this patient, given that the contralateral iliac arterial segment showed mild disease; hence, it was not considered as the best inflow to support an extra-anatomic cross-over bypass. After adequate heparinisation, the left CIA lesion was successfully treated with a balloon-expandable stent (Figures 2C and 2D; Express LD Iliac 8 mm: 4 cm; pressure limit, 11 atm; Boston Scientific), which spared the IIA, but a high inflation pressure balloon angioplasty after stenting (16 atm, 3 min) was required to relieve a stiff waist. Dual antiplatelet therapy (DAPT) was started along with atorvastatin. A distal trans-metatarsal amputation was carried out and the wound healed soundly. ABPI improved to 0.92 on the left side. Regular ABPI checks showed no further reductions bilaterally.
Case 2
A 55-year-old woman presented in 2016 with severe rest pain and dry right big toe gangrene of 10 days’ duration, 10 years after radiotherapy for Hodgkin’s lymphoma with a right inguinal lymph node biopsy. Over the 3 years before presentation, she had been admitted four times with severe right leg pain, swelling and bluish discolouration, and DVT was excluded each time on duplex scan (the most likely explanation was severe CVI due to iliac vein stenosis). Therefore, severe CVI was suggested, but none of these episodes were linked to the previous radiotherapy. Progressive lymphoedema had developed and the limb had attained a huge size. In addition, the patient had diabetes, hypertension, dyslipidaemia and ischaemic heart disease with coronary stenting.
On examination, the whole right lower limb was massively enlarged, the groin was indurated with light brown discolouration and there was a skin blister in the groin, near a lymph gland biopsy scar. There were multiple skin blisters and venous/lymphatic skin changes in the distal leg, in addition to the big toe dry gangrene. ABPI measurement was not possible because of the skin condition and limb swelling, but pedal waveforms were monophasic on the right side and biphasic on the left side, and toe brachial pressure index (TBPI) was 0.3 on the right side and 0.4 on the left side.
CT angiography (CTA) indicated bilateral mild tibial arterial multiple stenoses with short occluded segments in the tibio-peroneal trunks and a long atherosclerotic segment of stenoses and occlusions in the right SFA. The right CIA and external iliac artery were significantly narrowed with a sustained 7 cm segment of sub-occlusion, similar to a stretched thread, and very different from the SFA disease pattern. The right IIA was severely attenuated as well. The right common iliac vein (CIV) diameter was 6 mm and the diameter of the contralateral one was 8 mm. The diameter of the common femoral veins was 15 mm and 12 mm on the right and left sides, respectively.
Due to the multiple comorbidities and the nature of the disease, the endovascular option was considered to be the best treatment in this case. The right iliac arterial segment was accessed from the contralateral groin, after an IV heparin bolus, and two stents were deployed (Express LD Iliac 8 mm: 6 cm and 4 cm; pressure limit, 9 atm; Boston Scientific) with a 2 cm overlap. The SFA disease was treated with a RANGER paclitaxel-coated PTA balloon catheter on an 0.018" platform (100 mm, 6 mm diameter, 10 atm). Again, a higher than recommended inflation pressure limit was mandatory to adequately stretch the stent (16 atm). TBPI improved on the right side to 0.52 and waveforms became biphasic on the right side. DAPT was given along with a statin. Skincare instructions and class I compression stockings were advised to limit venous and lymphatic disease progression. Again, no further treatment was offered for the iliac veins disease because of the multiple comorbidities and the complexity of venolymphatic insufficiency.
Unfortunately, the patient was admitted to the intensive care unit 7 weeks after the intervention with septic shock due to severe necrotising fasciitis of the right lower limb. Above-the-knee amputation was unavoidable. Additionally, the right groin blister had worsened and developed into a classical radiation ulcer. It took almost 2 years for both wounds to heal.
Case 3
A 53-year-old woman presented in 2017 with progressively worsening severe rest pain and gangrene of the lateral three toes in her right foot, of 4 days duration. She had undergone endometrial cancer treatment with radiotherapy 9 years earlier. There was no history of angina, stroke or claudication. In addition, there was no clinical evidence of tumour recurrence.
On examination, all peripheral pulses were palpable in all extremities apart from the right lower limb. The right foot was colder than the left side and showed gangrene of the lateral three toes, along with skin mottling patches proximally, in addition to mild pitting oedema in the dorsal aspect of the foot with a sort of skin glistening. There was no cellulitis or deep collections. Her right groin was hard and indurated with a mahogany discolouration of the skin. ABPI was 0.3 on the right side and 1.1 on the left side. The clinical picture was a combination of acute and critical limb ischaemia; nonetheless, there was no motor impairment.
Urgent CTA showed a 6 cm uniform near-total occlusion of the distal CIA and proximal external iliac artery and the involved segment looked like a stretched thread, the distal flow was attenuated and no collaterals were noted. There was no radiological evidence of thrombosis. Otherwise, the whole arterial tree proximally and on the contralateral side was essentially unremarkable.
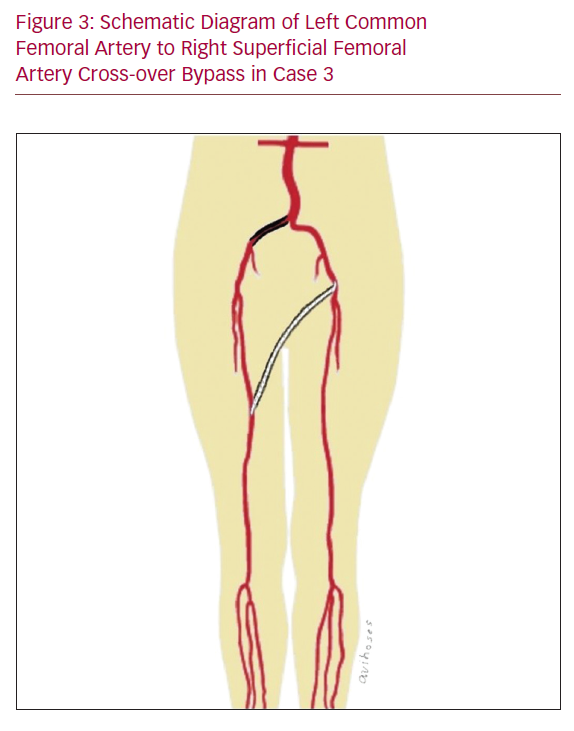
Urgent cross-over bypass was done given that there was no access to the out-of-hours interventional radiology service. Additionally, the disease morphology was considered as ideal for a short bypass, which was done with an inflow from the left CFA to the right SFA using a fluoropolymer-coated Dacron 6 mm graft to avoid dissection through the right groin (Figure 3).
Postoperatively, the foot condition improved, with an ABPI of 0.89 on the right side and 1.12 on the left side. Local foot amputation of the lateral three toes was carried out. The wound healing process was uneventful. The same treatment and follow-up protocols as in the previous cases were adopted in this case.
Case 4
A 67-year-old woman presented in 2017 with a 2 week history of fifth right toe tip gangrene and increasingly worsening leg ulcer over the previous 3 months, most likely a mixed arterial and venous ulcer. She had received radiotherapy for Hodgkin’s lymphoma, 12 years earlier, in addition to right groin inguinal lymph node biopsy. She had a background history of stable angina, diabetes and right hip osteoarthritis with bilateral knee mild flexion deformity. The right foot had only a dry gangrenous patch on the fifth toe. Also, the leg had a deep 4 × 5 cm ulcer above the medial malleolus, in addition to the knee mild contracture and the groin scar. ABPI was 0.5 on the right and 0.93 on the left .
CTA showed calcifications in different segments on both sides, but there were stenoses only on the right side; one tight stenotic segment in the distal 3 cm segment of the CIA; and atherosclerotic multiple stenoses in the SFA. Of note, the right CIV was narrower than the left CIV in cut sections distal to L5 (8 mm versus 13 mm in diameter, respectively), but not much information about the interior was obtainable from this arterial phase scan.
This patient was treated in a similar way to the previous case, given that she declined angioplasty because she was very conscious about the risk of renal impairment and dialysis, but the distal anastomosis was done at the retrogenicular popliteal segment. ABPI improved postoperatively to 0.85 on the right side, the patient was maintained on DAPT and a statin and was advised to have regular ABPI measurement. Moreover, multi-layer compression bandaging was necessary for 3 months to achieve complete healing of the leg ulcer.
Conclusion
The incidence of RIPVD is much higher than expected and can be easily missed. Hence, a high index of suspicion is the key to successful diagnosis. It should not be dealt with in a similar way to atherosclerotic arteriopathy. It seems that there is some female predilection to RIPVD.
In the presence of new lower limb ischaemic features with a background of pelvic radiotherapy, particularly with better prognosis tumours, iliac radiation arteritis should be borne in mind. Similarly, the picture of DVT or CVI in this setting should warrant detailed investigations to uncover the underlying radiation-related iliac vein disease.
Regardless of presentation, it is vital to consider other structures in the radiation field, for example major veins, lymphatics and pelvic viscera in imaging plans. In a high proportion of cases, radiation arteritis lesions can be easily differentiated from the atherosclerotic disease. However, this would be increasingly difficult in late lesions.
The outcome of limb salvage (surgical and interventional) procedures can be very variable and adequate planning is necessary to reduce the risk of major amputation. Every effort should be made to avoid surgical manipulation of the radiation field. Follow-up after a successful procedure is needed in all cases, indefinitely, given that late radiation tissue injury is an ongoing process.1,2,13–17,29